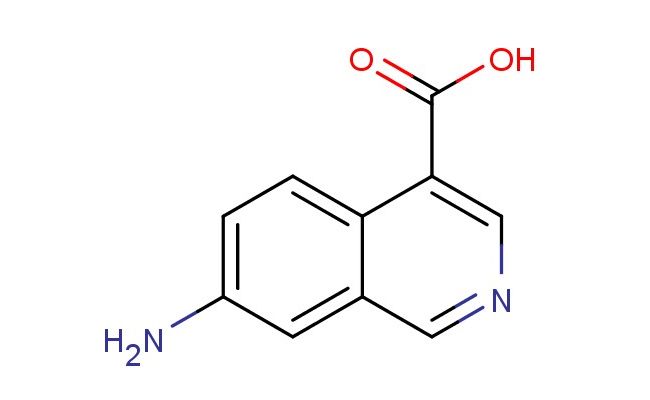
7-aminoisoquinoline-4-carboxylic acid
$550.00
CAS No.: 1934945-04-6
Catalog No.: 191978
Purity: 95%
MF: C10H8N2O2
MW: 188.186
Storage: 2-8 degree Celsius
SMILES: NC1=CC=C2C(=CN=CC2=C1)C(=O)O
Catalog No.: 191978
Purity: 95%
MF: C10H8N2O2
MW: 188.186
Storage: 2-8 degree Celsius
SMILES: NC1=CC=C2C(=CN=CC2=C1)C(=O)O
7-aminoisoquinoline-4-carboxylic acid; CAS No.: 1934945-04-6;7-aminoisoquinoline-4-carboxylic acid. PROPERTIES: 7-aminoisoquinoline-4-carboxylic acid is a heterocyclic amino acid with molecular formula C10H7N3O2. This crystalline compound typically appears as white powder and has a melting point between 230-235 C. The molecule features an isoquinoline core with an amine group at position 7 and a carboxylic acid group at position 4. The 7-aminoisoquinoline-4-carboxylic acid exhibits zwitterionic character under physiological conditions, with a pKa value around 3.2 for the carboxylic acid group and around 9.8 for the amine group. It shows good solubility in water and polar organic solvents. Proper storage requires maintaining in a tightly sealed container with appropriate drying agents, stored below 20 C to prevent hydrolytic degradation. Safety measures include using P295 respiratory protection and chemical-resistant gloves, as the compound may cause respiratory irritation and skin sensitization. According to GHS classification, it carries H317 and H335 hazard statements for potential sensitization and respiratory tract irritation. APPLICATIONS: The 7-aminoisoquinoline-4-carboxylic acid structure functions as a key intermediate in the synthesis of isoquinoline-based dipeptidyl peptidase-4 (DPP-4) inhibitors, as reported in medicinal chemistry research focusing on antidiabetic agents. The amino and carboxylic acid groups enable peptide coupling reactions to form bioactive conjugates. Additionally, 7-aminoisoquinoline-4-carboxylic acid derivatives have been explored in materials science for creating electroactive polymers with interesting optical properties, as described in polymer chemistry publications. The compound's ability to participate in hydrogen bonding interactions has also made it valuable in developing molecular receptors for anion recognition, as documented in supramolecular chemistry studies emphasizing host-guest chemistry.
Reviews
Write Your Own Review