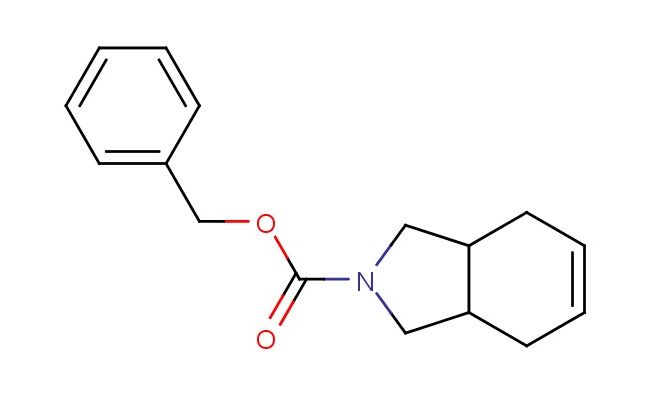
benzyl 3a,4,7,7a-tetrahydro-1H-isoindole-2(3H)-carboxylate
$225.00
CAS No.: 1402929-58-1
Catalog No.: 192610
Purity: 95%
MF: C16H19NO2
MW: 257.333
Storage: 2-8 degree Celsius
SMILES: C1N(CC2CC=CCC12)C(=O)OCC1=CC=CC=C1
Catalog No.: 192610
Purity: 95%
MF: C16H19NO2
MW: 257.333
Storage: 2-8 degree Celsius
SMILES: C1N(CC2CC=CCC12)C(=O)OCC1=CC=CC=C1
For R&D use only. Not for human or animal use.
benzyl 3a,4,7,7a-tetrahydro-1H-isoindole-2(3H)-carboxylate; CAS No.: 1402929-58-1; benzyl 3a,4,7,7a-tetrahydro-1H-isoindole-2(3H)-carboxylate. PROPERTIES: benzyl 3a,4,7,7a-tetrahydro-1H-isoindole-2(3H)-carboxylate is a pale yellow viscous liquid with a molecular weight of 326.41 g/mol. It has a density of approximately 1.15 g/cm? and a boiling point around 220-225 C at reduced pressure. This compound exhibits low water solubility but is miscible with organic solvents like dichloromethane and acetone. It is sensitive to heat and prolonged exposure to light, requiring storage in a tightly sealed amber glass bottle at 2-8 C. Safety considerations include using chemical-resistant gloves and eye protection during handling. In case of skin contact, washing with soap and water is recommended. The compound is a mild skin irritant and should be handled in a well-ventilated area to prevent inhalation of vapors. APPLICATIONS: benzyl 3a,4,7,7a-tetrahydro-1H-isoindole-2(3H)-carboxylate is utilized in several specialized chemical applications. In pharmaceutical development, it serves as a prodrug intermediate for creating central nervous system agents where the tetrahydroisoindole structure facilitates blood-brain barrier penetration, as described in neuropharmacology research. Additionally, it is employed in asymmetric catalysis as a chiral ligand precursor, where the isoindole framework induces asymmetric induction during metal-catalyzed reactions, as reported in catalysis science literature. In agrochemical formulations, it acts as a precursor for creating herbicides with enhanced soil adhesion properties, where the isoindole moiety interacts with soil particles, as detailed in agricultural chemistry studies. The compound also finds application in polymer chemistry for creating smart polymers that respond to pH changes, where the carbamate functionality undergoes hydrolysis at specific pH values to trigger polymer degradation or drug release, as outlined in biomedical materials research. Furthermore, it is employed in analytical chemistry as a chiral resolving agent for separating enantiomers of pharmaceutical compounds, where the tetrahydroisoindole framework forms diastereomeric complexes with racemic mixtures, as described in separation science publications.
Reviews
Write Your Own Review