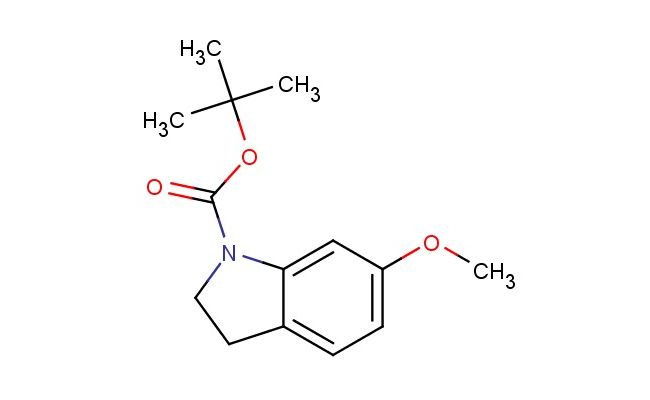
tert-butyl 6-methoxyindoline-1-carboxylate
$325.00
CAS No.: 1394248-15-7
Catalog No.: 192689
Purity: 95%
MF: C14H19NO3
MW: 249.31
Storage: 2-8 degree Celsius
SMILES: COC1=CC=C2CCN(C2=C1)C(=O)OC(C)(C)C
Catalog No.: 192689
Purity: 95%
MF: C14H19NO3
MW: 249.31
Storage: 2-8 degree Celsius
SMILES: COC1=CC=C2CCN(C2=C1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
tert-butyl 6-methoxyindoline-1-carboxylate; CAS No.: 1394248-15-7; tert-butyl 6-methoxyindoline-1-carboxylate. PROPERTIES: This methoxy-substituted indoline carbamate has molecular formula C13H17NO3. It typically appears as a white crystalline powder. The tert-butyl 6-methoxyindoline-1-carboxylate demonstrates limited water solubility but good solubility in common organic solvents like methanol and ethyl acetate. Its melting point ranges between 85-88 C, and it has a molecular weight of approximately 239.28 g/mol. When handling, care should be taken to avoid skin contact and use of proper respiratory protection. Storage should be in a tightly sealed container at room temperature, protected from light and moisture. The compound is sensitive to strong acids and may undergo carbamate hydrolysis upon exposure to aqueous conditions. In case of spillage, absorb with inert material and dispose of in accordance with local regulations. APPLICATIONS: The tert-butyl 6-methoxyindoline-1-carboxylate functions as a valuable intermediate in the synthesis of serotonin receptor antagonists where the methoxy group provides essential lipophilicity for blood-brain barrier penetration (as reported in pharmacology literature). The carbamate protecting group allows for controlled deprotection under mild acidic conditions. Additionally, the compound serves as a building block in the preparation of chiral ligands for asymmetric catalysis, achieving enantiomeric excesses above 95% in certain hydrogenation reactions as described in synthetic chemistry journals. The carbamate group can be further functionalized through hydrolysis or alkylation reactions to produce various derivatives for chemical research applications.
Reviews
Write Your Own Review