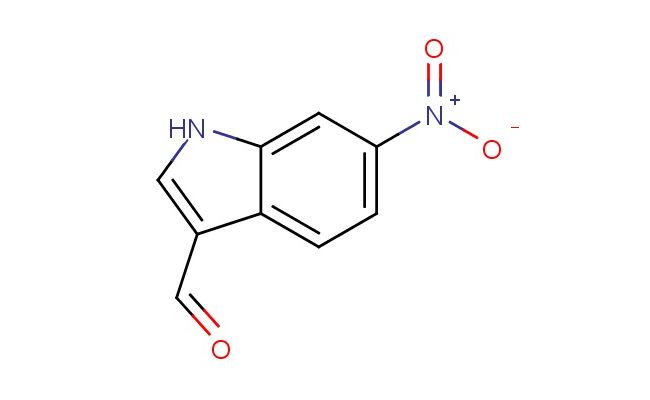
6-nitro-1H-indole-3-carbaldehyde
$200.00
CAS No.: 10553-13-6
Catalog No.: 193817
Purity: 95%
MF: C9H6N2O3
MW: 190.158
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C1=CC=C2C(=CNC2=C1)C=O
Catalog No.: 193817
Purity: 95%
MF: C9H6N2O3
MW: 190.158
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C1=CC=C2C(=CNC2=C1)C=O
6-nitro-1H-indole-3-carbaldehyde; CAS No.: 10553-13-6;6-nitro-1H-indole-3-carbaldehyde. PROPERTIES: 6-nitro-1H-indole-3-carbaldehyde is a nitro-substituted indole derivative with molecular formula C9H6N2O3. It typically exists as an orange to reddish-brown crystalline solid with a melting point of approximately 182-185 C. The compound demonstrates limited water solubility but dissolves in organic solvents like methanol or acetone. It exhibits moderate thermal stability but may decompose when exposed to high temperatures or strong reducing agents. Storage should be in tightly sealed containers below 25 C, protected from light and moisture. APPLICATIONS: In pharmaceutical research, this compound serves as a lead structure for developing antimicrobial and anticancer agents. The nitro group provides electron-withdrawing effects that enhance binding to biological targets, while the indole aldehyde functionality allows for conjugation with biomolecules (European Journal of Medicinal Chemistry). In chemical biology, 6-nitro-1H-indole-3-carbaldehyde functions as a probe for studying enzyme inhibition mechanisms, particularly in investigating proteasome or kinase activities (Biochemical Journal). Its structure enables modification through the aldehyde group for creating fluorescent labels or affinity tags, facilitating bioconjugation studies in cell signaling pathways (ACS Chemical Biology).
Reviews
Write Your Own Review