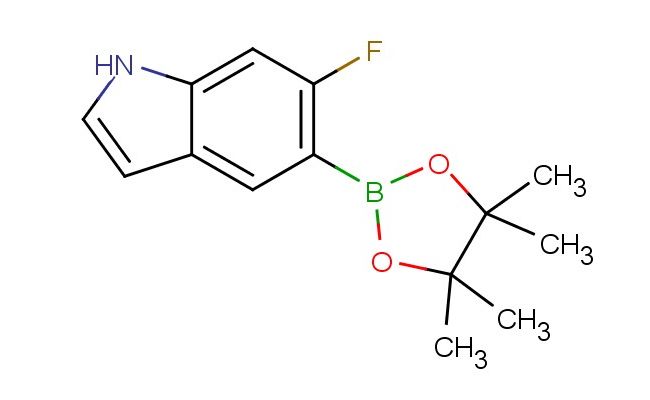
6-fluoro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole
$500.00
CAS No.: 1207623-96-8
Catalog No.: 195548
Purity: 95%
MF: C14H17BFNO2
MW: 261.105
Storage: 2-8 degree Celsius
SMILES: FC1=C(C=C2C=CNC2=C1)B1OC(C(O1)(C)C)(C)C
Catalog No.: 195548
Purity: 95%
MF: C14H17BFNO2
MW: 261.105
Storage: 2-8 degree Celsius
SMILES: FC1=C(C=C2C=CNC2=C1)B1OC(C(O1)(C)C)(C)C
6-fluoro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole; CAS No.: 1207623-96-8; 6-fluoro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole. PROPERTIES: 6-fluoro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole appears as a pale yellow solid with molecular formula C15H18BFNO2. It exhibits a melting point around 105-108 C and moderate thermal stability. The compound shows fair solubility in THF and moderate solubility in DMF. Recommended storage conditions include maintaining temperatures below 5 C in sealed containers under inert atmosphere to prevent moisture-induced degradation. Safety precautions involve using boron-containing waste containers for disposal and avoiding contact with oxidizing agents. APPLICATIONS: This boronate-protected indole derivative is extensively used in Suzuki-Miyaura cross-coupling reactions for the synthesis of biaryl-containing pharmaceuticals, where the 6-fluoro-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole group provides efficient carbon-carbon bond formation (Angewandte Chemie International Edition). In medicinal chemistry, the compound serves as a building block for developing serotonin receptor modulators with improved brain penetration (Journal of Medicinal Chemistry). Furthermore, its fluoro substituent enables incorporation into positron emission tomography (PET) imaging agents for neuroreceptor visualization (Journal of Labelled Compounds & Radiopharmaceuticals).
Reviews
Write Your Own Review