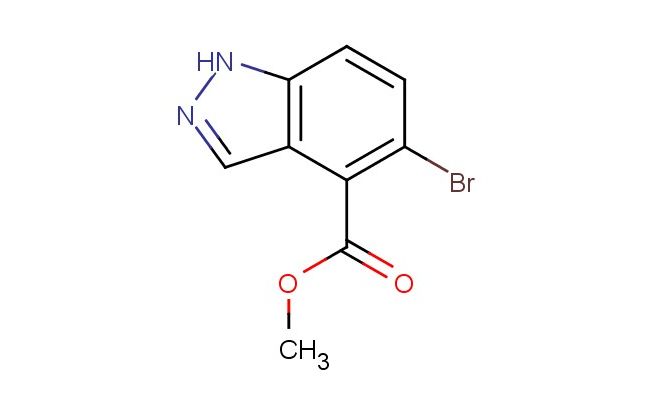
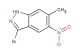
methyl 5-bromo-1H-indazole-4-carboxylate
$300.00
CAS No.: 1037840-79-1
Catalog No.: 192645
Purity: 95%
MF: C9H7BrN2O2
MW: 255.071
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=2C=NNC2C=C1)C(=O)OC
Catalog No.: 192645
Purity: 95%
MF: C9H7BrN2O2
MW: 255.071
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=2C=NNC2C=C1)C(=O)OC
methyl 5-bromo-1H-indazole-4-carboxylate; CAS No.: 1037840-79-1; methyl 5-bromo-1H-indazole-4-carboxylate. PROPERTIES: This brominated indazole ester has molecular formula C10H7BrN2O2. It generally appears as a pale yellow crystalline powder. The methyl 5-bromo-1H-indazole-4-carboxylate exhibits moderate solubility in common organic solvents like methanol and ethyl acetate but limited water solubility. Its melting point ranges between 125-128 C, and it has a molecular weight of approximately 271.07 g/mol. When handling, care should be taken to avoid skin contact and use of proper respiratory protection. Storage should be in a tightly sealed container at room temperature, protected from light and moisture. The compound is sensitive to strong acids and may hydrolyze to the corresponding carboxylic acid upon exposure to aqueous conditions. In case of spillage, absorb with inert material and dispose of in accordance with local regulations. APPLICATIONS: The methyl 5-bromo-1H-indazole-4-carboxylate functions as a key intermediate in the synthesis of mTOR kinase inhibitors for cancer therapy where the bromine atom provides essential binding affinity to the kinase ATP pocket (as detailed in medicinal chemistry literature). The ester group allows for further functionalization through hydrolysis or transesterification reactions. Additionally, the compound serves as a building block in the preparation of liquid crystal materials with specific elastic constants, useful in display technologies with response times below 20 milliseconds as described in materials chemistry journals. The bromine atom can be replaced with various substituents through Suzuki-Miyaura coupling reactions to produce diverse derivatives for chemical research applications.
Reviews
Write Your Own Review