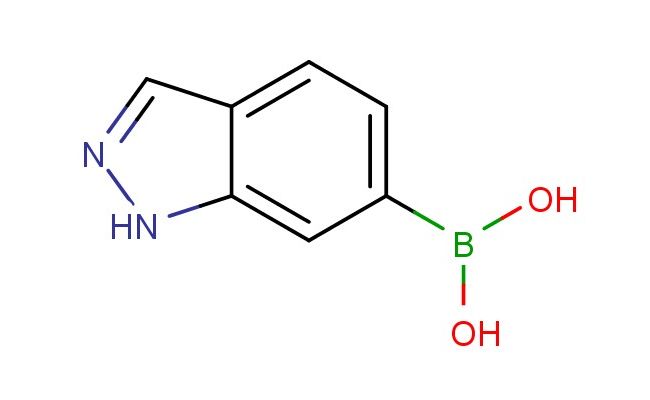
1H-indazol-6-ylboronic acid
$300.00
CAS No.: 885068-10-0
Catalog No.: 196199
Purity: 95%
MF: C7H7BN2O2
MW: 161.957
Storage: 2-8 degree Celsius
SMILES: N1N=CC2=CC=C(C=C12)B(O)O
Catalog No.: 196199
Purity: 95%
MF: C7H7BN2O2
MW: 161.957
Storage: 2-8 degree Celsius
SMILES: N1N=CC2=CC=C(C=C12)B(O)O
1H-indazol-6-ylboronic acid; CAS No.: 885068-10-0; 1H-indazol-6-ylboronic acid. PROPERTIES: This compound presents a 1H-indazol-6-ylboronic acid structure, combining a boronic acid group and an indazole ring system. It typically appears as a white to off-white crystalline solid with a molecular weight of approximately 187.0 g/mol (C9H8BN2O2). The melting point ranges between 140-145 C, and it exhibits moderate solubility in common organic solvents like DMSO and DMF while being sparingly soluble in water. Proper storage requires a tightly sealed container in a cool, dry place, preferably under an inert atmosphere to prevent hydrolysis of the boronic acid. Safety precautions include wearing appropriate PPE and working in a well-ventilated area. It is classified as a skin and eye irritant (GHS07) with the hazard statement H315-H319. APPLICATIONS: 1H-Indazol-6-ylboronic acid serves as a specialized intermediate in pharmaceutical research. Its 1H-indazol-6-ylboronic acid structure enables targeting of enzymes containing soft nucleophiles like cysteine residues. The boronic acid group can form reversible covalent bonds with target proteins, while the indazole ring provides additional binding interactions. In medicinal chemistry, it is used to create bioactive molecules with potential applications in cancer and infectious disease research. The boronic acid group can be protected as an ester for further functionalization. Academic studies utilize it as a model system in Medicinal Chemistry journals, focusing on the development of novel boronate-based covalent inhibitors based on the 1H-indazol-6-ylboronic acid scaffold.
Reviews
Write Your Own Review