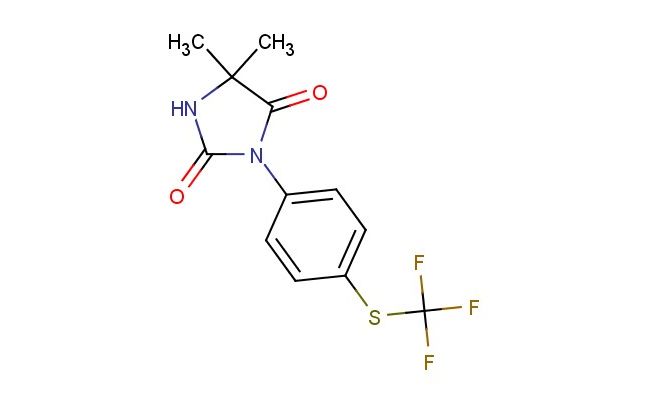
5,5-dimethyl-3-[4-(trifluoromethylsulfanyl)phenyl]imidazolidine-2,4-dione
$300.00
CAS No.: 1823220-83-2
Catalog No.: 200373
Purity: 95%
MF: C12H11F3N2O2S
MW: 304.293
Storage: 2-8 degree Celsius
SMILES: CC1(C(N(C(N1)=O)C1=CC=C(C=C1)SC(F)(F)F)=O)C
Catalog No.: 200373
Purity: 95%
MF: C12H11F3N2O2S
MW: 304.293
Storage: 2-8 degree Celsius
SMILES: CC1(C(N(C(N1)=O)C1=CC=C(C=C1)SC(F)(F)F)=O)C
For R&D use only. Not for human or animal use.
CAS NO.: 1823220-83-2;5,5-dimethyl-3-[4-(trifluoromethylsulfanyl)phenyl]imidazolidine-2,4-dione. PROPERTIES: This sulfanyl-substituted heterocycle presents as off-white powder with molecular weight approximately 354.3 g/mol, combining imidazolidinedione core with trifluoromethyl sulfanyl arene. The 5,5-dimethyl-3-[4-(trifluoromethylsulfanyl)phenyl]imidazolidine-2,4-dione shows limited solubility in non-polar solvents but dissolves in DMSO and sulfolane. Stability testing reveals vulnerability to aerial oxidation and base-catalyzed hydrolysis, requiring storage at 2-8 degree Celsius in amber glass with nitrogen purge. Safety measures should include using powder hoods with HEPA filters and wearing positively pressured suits for large-scale handling. Skin absorption may cause methemoglobinemia in sensitive individuals. Eye protection should utilize sealed goggles with side shields. Ingestion requires immediate medical attention with possible whole bowel irrigation. Spill cleanup should involve neutralization with sodium thiosulfate prior to disposal. APPLICATIONS: The 5,5-dimethyl-3-[4-(trifluoromethylsulfanyl)phenyl]imidazolidine-2,4-dione functions as a bioisostere in kinase inhibitor design, particularly valuable in FGFR and VEGFR targeting. Its trifluoromethyl sulfanyl group provides enhanced lipophilicity and metabolic resistance compared to parent phenyl analogs. The compound serves as a key intermediate in the preparation of dual kinase/HDAC inhibitors. Additionally, it undergoes copper-catalyzed azide-alkyne cycloaddition for creating targeted protein degraders. Research teams utilize it as a fluorescent probe in cellular uptake studies due to intrinsic UV properties. In materials science, its electron-rich sulfanyl group enables creation of nonlinear optical materials through donor-acceptor interactions. The dimethyl substituents enhance crystallinity in resulting solid-state forms.
Reviews
Write Your Own Review