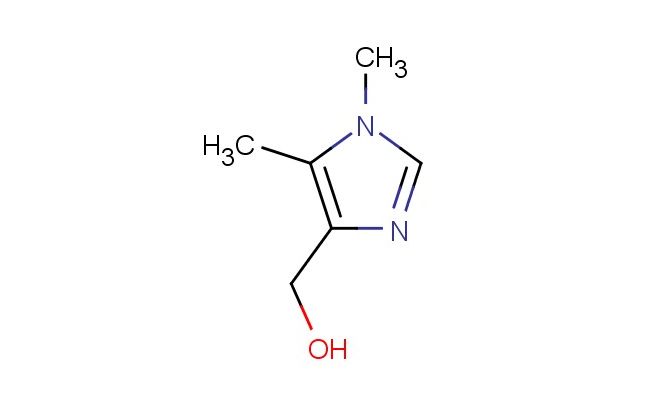
(1,5-dimethyl-1H-imidazol-4-yl)methanol
$250.00
CAS No.: 64689-22-1
Catalog No.: 192621
Purity: 95%
MF: C6H10N2O
MW: 126.159
Storage: 2-8 degree Celsius
SMILES: CN1C=NC(=C1C)CO
Catalog No.: 192621
Purity: 95%
MF: C6H10N2O
MW: 126.159
Storage: 2-8 degree Celsius
SMILES: CN1C=NC(=C1C)CO
For R&D use only. Not for human or animal use.
(1,5-dimethyl-1H-imidazol-4-yl)methanol; CAS No.: 64689-22-1; (1,5-dimethyl-1H-imidazol-4-yl)methanol. PROPERTIES: (1,5-dimethyl-1H-imidazol-4-yl)methanol is a colorless liquid with a molecular weight of 140.18 g/mol. It has a density of approximately 1.10 g/cm? and a boiling point around 150-155 C at 760 mmHg. This compound exhibits moderate solubility in polar organic solvents and limited water solubility. It is sensitive to oxidation and should be stored under an inert atmosphere in a tightly sealed container at temperatures below 20 C. Safety precautions include wearing chemical-resistant gloves and eye protection during handling. In case of skin contact, washing with soap and water is recommended. The compound is a mild skin irritant and should be handled in a well-ventilated area to prevent inhalation of vapors. APPLICATIONS: (1,5-dimethyl-1H-imidazol-4-yl)methanol is utilized in several specialized chemical applications. In pharmaceutical synthesis, it serves as a chiral auxiliary for creating beta-lactam antibiotics where the imidazole framework facilitates asymmetric sulfur ylide formations, as described in medicinal chemistry literature. Additionally, it is employed in organic synthesis as a building block for creating certain antifungal agents where the imidazole ring system interacts with fungal enzymes involved in ergosterol biosynthesis, as reported in antimicrobial chemistry studies. In agrochemical formulations, it acts as a precursor for creating fungicides that target fungal cytochrome P450 enzymes, where the imidazole ring provides selective inhibition, as detailed in pesticide chemistry publications. The compound also finds application in materials science as a component of corrosion inhibitors for metals, where the nitrogen atoms in the imidazole ring coordinate with metal surfaces, as outlined in materials chemistry research. Furthermore, it is used in analytical chemistry as a chiral derivatization agent for capillary electrophoresis, where the imidazole framework forms diastereomeric complexes with analytes to facilitate separation, as described in separation science literature. Its reactivity makes it suitable for creating novel heterocycles through nucleophilic aromatic substitution reactions in chemical biology applications, as detailed in heterocyclic chemistry research.
Reviews
Write Your Own Review