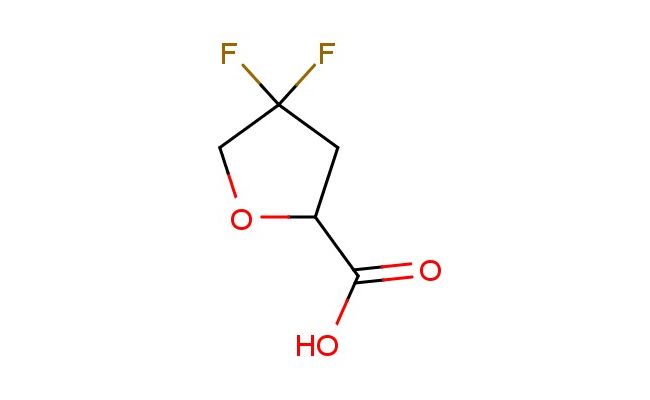
4,4-difluorotetrahydrofuran-2-carboxylic acid
$700.00
CAS No.: 2056072-61-6
Catalog No.: 200404
Purity: 95%
MF: C5H6F2O3
MW: 152.096
Storage: 2-8 degree Celsius
SMILES: FC1(CC(OC1)C(=O)O)F
Catalog No.: 200404
Purity: 95%
MF: C5H6F2O3
MW: 152.096
Storage: 2-8 degree Celsius
SMILES: FC1(CC(OC1)C(=O)O)F
For R&D use only. Not for human or animal use.
CAS NO.: 2056072-61-6;4,4-difluorotetrahydrofuran-2-carboxylic acid. PROPERTIES: This difluorinated heterocyclic acid presents as colorless needles with molecular weight approximately 168.1 g/mol, combining tetrahydrofuran ring strain with vicinal difluoro and carboxylic acid groups. The 4,4-difluorotetrahydrofuran-2-carboxylic acid exhibits limited aqueous solubility until pH < 2 but good dissolution in acetic acid and DMF. Stability testing reveals vulnerability to acid-catalyzed ring-opening and base-promoted tautomerism, necessitating storage at 2-8 degree Celsius in borosilicate glass. Handlers should employ resistive gloves when handling concentrated solutions to prevent dermal absorption. Inhalation may cause metabolic acidosis; treatment includes alkaline inhalations. Eye contact requires prolonged rinsing due to potential for persistent corneal staining. Waste neutralization should be conducted at pH 8-9 to minimize ring-opening degradation. APPLICATIONS: The 4,4-difluorotetrahydrofuran-2-carboxylic acid functions as a key intermediate in the synthesis of prostaglandin analogs and COX-2 selective inhibitors. Its difluorotetrahydrofuran framework provides enhanced metabolic stability compared to parent tetrahydrofuran analogs. The compound serves as a building block for creating macrocyclic kinase inhibitors through lactonization reactions. Additionally, it undergoes Stille reaction for creating fused bicyclic systems. Research teams utilize it as a starting material for creating tetrahydrofuran-based fluorescent probes. In materials science, its carboxylic acid group enables formation of self-assembled monolayers with tuned wettability.
Reviews
Write Your Own Review