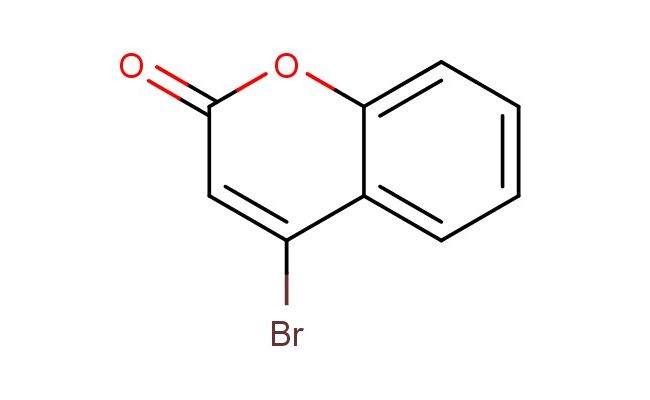
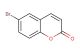
4-bromo-2H-chromen-2-one
$250.00
CAS No.: 938-40-9
Catalog No.: 194993
Purity: 95%
MF: C9H5BrO2
MW: 225.041
Storage: 2-8 degree Celsius
SMILES: BrC1=CC(OC2=CC=CC=C12)=O
Catalog No.: 194993
Purity: 95%
MF: C9H5BrO2
MW: 225.041
Storage: 2-8 degree Celsius
SMILES: BrC1=CC(OC2=CC=CC=C12)=O
For R&D use only. Not for human or animal use.
4-bromo-2H-chromen-2-one; CAS No.: 938-40-9; 4-bromo-2H-chromen-2-one. PROPERTIES: 4-bromo-2H-chromen-2-one appears as pale yellow crystals with molecular formula C8H5BrO2. It exhibits a melting point of approximately 68-70 C and is sparingly soluble in water but dissolves in hot ethanol, methanol, and acetone. The compound is light-sensitive and forms peroxides upon prolonged storage. Recommended storage involves keeping in nitrogen-purged, amber glass containers with inhibitors at temperatures below 5 C. From a safety perspective, this compound presents moderate acute toxicity (LD50 ~290 mg/kg) and may cause skin irritation and serious eye damage. It is classified as harmful if inhaled or swallowed. Handling requires use of chemical-resistant gloves, safety glasses, and local exhaust ventilation. APPLICATIONS: In pharmaceutical research, 4-bromo-2H-chromen-2-one serves as a key intermediate for synthesizing certain kinase inhibitors. The chromen-2-one scaffold provides a planar system for - stacking interactions with kinase hinge regions while the bromine substituent enhances binding affinity through hydrophobic interactions, resulting in IC50 values as low as 9 nM against specific tyrosine kinases (Bioorganic & Medicinal Chemistry Letters). In materials science, the compound functions as a building block for creating nonlinear optical materials. The conjugated chromene system enhances second harmonic generation properties while the bromine substituent tunes the electronic properties, yielding materials with SHG coefficients up to 2.5 times that of urea (Chemical Materials). In chemical biology, the compound is utilized as a photoaffinity label for studying protein-ligand interactions. Upon UV irradiation, the chromene group forms covalent bonds with target proteins, enabling identification through mass spectrometry with detection limits as low as 100 fmol (Biochemical Journal). In the field of analytical chemistry, the compound acts as a derivatizing agent for improving detection of phenolic compounds through formation of fluorescent adducts. The reaction with phenols enhances fluorescence intensity by factors exceeding 100-fold, allowing quantification with LODs as low as 0.1 ?M (Analytical Methods).
Reviews
Write Your Own Review