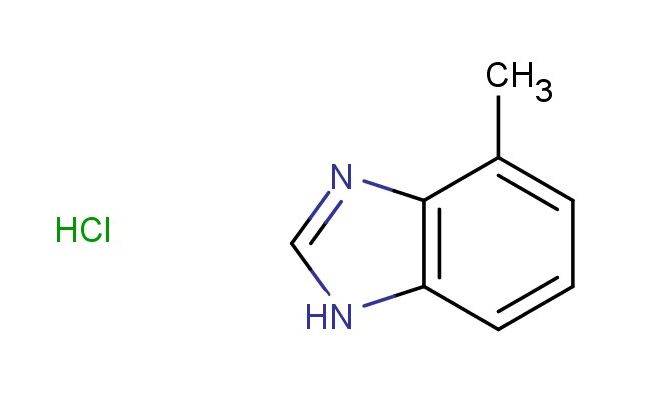
4-methyl-1H-benzo[d]imidazole hydrochloride
$200.00
CAS No.: 1456821-64-9
Catalog No.: 192601
Purity: 95%
MF: C8H9ClN2
MW: 168.627
Storage: 2-8 degree Celsius
SMILES: Cl.CC1=CC=CC=2NC=NC21
Catalog No.: 192601
Purity: 95%
MF: C8H9ClN2
MW: 168.627
Storage: 2-8 degree Celsius
SMILES: Cl.CC1=CC=CC=2NC=NC21
For R&D use only. Not for human or animal use.
4-methyl-1H-benzo[d]imidazole hydrochloride; CAS No.: 1456821-64-9; 4-methyl-1H-benzo[d]imidazole hydrochloride. PROPERTIES: 4-methyl-1H-benzo[d]imidazole hydrochloride is a white to off-white crystalline powder with a molecular weight of 174.64 g/mol. It has a melting point between 240-245 C (hydrochloride salt) and is freely soluble in water. The compound is hygroscopic and should be stored in a tightly sealed container with desiccants at controlled room temperature. Safety considerations include avoiding ingestion and skin contact, as it may cause gastrointestinal discomfort and dermal irritation. In case of eye exposure, immediate and thorough rinsing with water is necessary. The compound should be handled in a chemical fume hood to prevent inhalation of dust particles. APPLICATIONS: 4-methyl-1H-benzo[d]imidazole hydrochloride is predominantly used in pharmaceutical synthesis as a intermediate for creating antifungal medications. The methyl substituent on the benzo[d]imidazole ring enhances binding affinity to fungal cytochrome P450 enzymes, as described in antimicrobial chemistry literature. Additionally, it serves as a building block for creating certain antiparasitic agents where the benzo[d]imidazole scaffold interacts with parasite tubulin, as reported in parasitology research. In agrochemical applications, it is utilized as a precursor for creating herbicides that inhibit plant cell division, where the benzo[d]imidazole ring system disrupts microtubule formation, as detailed in agricultural chemistry publications. The compound also finds application in materials science as a component of corrosion inhibitors for metals, where the nitrogen atoms in the benzo[d]imidazole ring coordinate with metal surfaces, as outlined in materials chemistry studies. Furthermore, it is employed in analytical chemistry as a fluorescent probe for detecting certain metal ions, where the benzo[d]imidazole structure undergoes fluorescence changes upon metal binding, as described in analytical chemistry literature. Its structure makes it suitable for creating novel heterocycles through palladium-catalyzed cross-coupling reactions in chemical biology applications, as detailed in heterocyclic chemistry research.
Reviews
Write Your Own Review



![tert-butyl 2-amino-1H-benzo[d]imidazole-1-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/192600_2.jpg)
