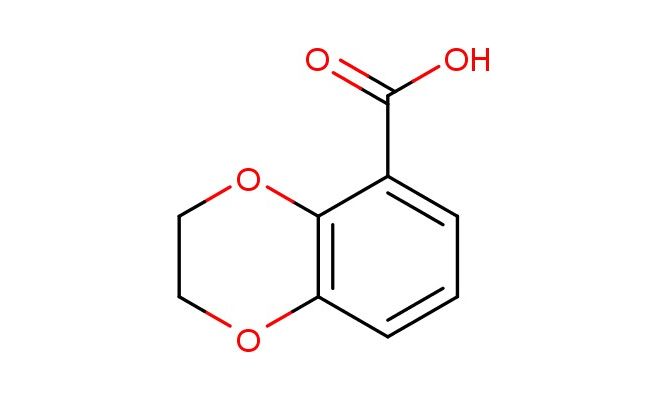
2,3-dihydrobenzo[b][1,4]dioxine-5-carboxylic acid
$250.00
CAS No.: 4442-53-9
Catalog No.: 196140
Purity: 95%
MF: C9H8O4
MW: 180.159
Storage: 2-8 degree Celsius
SMILES: O1C2=C(OCC1)C(=CC=C2)C(=O)O
Catalog No.: 196140
Purity: 95%
MF: C9H8O4
MW: 180.159
Storage: 2-8 degree Celsius
SMILES: O1C2=C(OCC1)C(=CC=C2)C(=O)O
For R&D use only. Not for human or animal use.
2,3-dihydrobenzo[b][1,4]dioxine-5-carboxylic acid; CAS No.: 4442-53-9; 2,3-dihydrobenzo[b][1,4]dioxine-5-carboxylic acid. PROPERTIES: This white to pale yellow crystalline powder has a molecular formula of C8H8O3 and a molecular weight of approximately 156.15 g/mol. It exhibits moderate solubility in water and common polar solvents. The compound is sensitive to basic conditions and should be stored in a tightly sealed container at room temperature. Thermogravimetric analysis shows decomposition starting at 200 C. Safety precautions include using chemical-resistant gloves, safety goggles, and working in a well-ventilated area. In case of accidental ingestion, rinse mouth and seek immediate medical advice. Avoid release to the environment as it may be harmful to aquatic organisms. APPLICATIONS: 2,3-dihydrobenzo[b][1,4]dioxine-5-carboxylic acid functions as a versatile intermediate in pharmaceutical synthesis, particularly in the development of kinase inhibitors and GPCR modulators. Its dioxane core provides conformational constraints useful in controlling bioactive conformations. The carboxylic acid group enables further functionalization through amide bond formation or esterification. Research groups employ it in the development of HCV NS5B polymerase inhibitors for antiviral applications. Academic institutions utilize it in teaching medicinal chemistry and bioorganic chemistry principles. Industrial applications include its use as a building block in agrochemical development for novel herbicide candidates targeting enzyme inhibition. Recent studies in the Journal of Medicinal Chemistry demonstrate its application in developing dual kinase/HDAC inhibitors. Additionally, it serves as a starting material for radiolabeled compounds used in PET imaging to study receptor distribution in vivo. The compound's ability to form hydrogen bonds makes it suitable for crystallographic studies of protein-ligand complexes. Its synthetic versatility enables rapid diversification through various carboxylic acid reactions and dioxane ring modifications.
Reviews
Write Your Own Review



![2,3-dihydrobenzo[b][1,4]dioxin-5-ol](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196139_2.jpg)
![7-nitro-2,3-dihydrobenzo[b][1,4]dioxine-6-carboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196142_2.jpg)