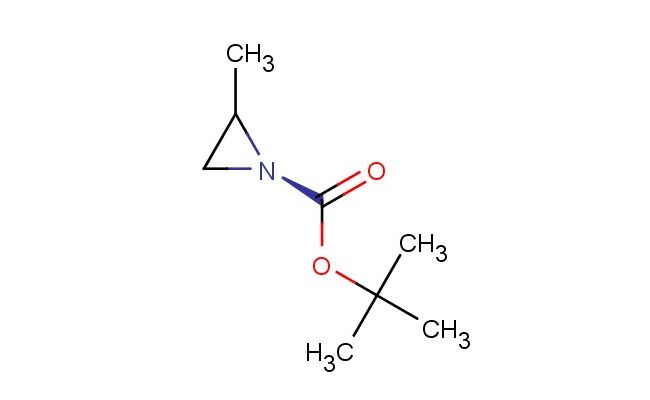
(S)-tert-Butyl 2-methylaziridine-1-carboxylate
$200.00
CAS No.: 197020-60-3
Catalog No.: 192586
Purity: 95%
MF: C8H15NO2
MW: 157.213
Storage: 2-8 degree Celsius
SMILES: CC1[N@](C1)C(=O)OC(C)(C)C
Catalog No.: 192586
Purity: 95%
MF: C8H15NO2
MW: 157.213
Storage: 2-8 degree Celsius
SMILES: CC1[N@](C1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
(S)-tert-Butyl 2-methylaziridine-1-carboxylate; CAS No.: 197020-60-3; (S)-tert-Butyl 2-methylaziridine-1-carboxylate. PROPERTIES: (S)-tert-Butyl 2-methylaziridine-1-carboxylate is a pale yellow liquid with a molecular weight of 173.22 g/mol. It has a density of approximately 1.00 g/cm? and a boiling point of approximately 95-100 C at reduced pressure. This compound is moderately soluble in non-polar organic solvents and has limited water solubility. It is sensitive to acidic conditions and hydrolyzes in strong acidic environments to release the corresponding amine. Proper storage requires a tightly sealed container in a cool, dark place, ideally at temperatures below 20 C. Safety considerations include avoiding inhalation of vapors, which may cause respiratory irritation. Skin contact should be minimized as it can lead to absorption and potential systemic effects. In case of eye exposure, immediate rinsing with water for at least 15 minutes is necessary. The compound should be handled using appropriate engineering controls to minimize vapor release. APPLICATIONS: (S)-tert-Butyl 2-methylaziridine-1-carboxylate is extensively used in the pharmaceutical industry as a chiral intermediate for creating enantiomerically pure compounds. Its aziridine ring is particularly valuable in synthesizing beta-blockers where the chiral center influences receptor specificity, as detailed in medicinal chemistry literature. Additionally, it serves as a key building block for creating chiral auxiliaries in asymmetric epoxidation reactions, where the methyl group on the aziridine ring directs stereochemistry during oxygen insertion, as reported in organic synthesis journals. In material science, it is utilized for creating chiral liquid crystals, where the aziridine motif contributes to spontaneous polarization and electro-optical properties, as described in liquid crystal technology studies. The compound also finds application in agrochemicals as a precursor for creating chiral fungicides, where the enantiomeric purity enhances bioactivity against specific fungal pathogens, as outlined in agricultural chemical research. Furthermore, it is employed in peptide synthesis as a non-natural amino acid surrogate, allowing for the creation of protease-resistant peptides with enhanced metabolic stability, as detailed in bioorganic chemistry publications.
Reviews
Write Your Own Review