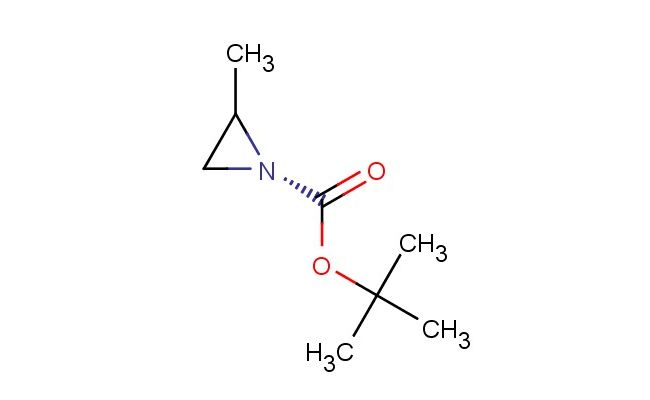
(R)-tert-Butyl 2-methylaziridine-1-carboxylate
$200.00
CAS No.: 129319-91-1
Catalog No.: 192585
Purity: 95%
MF: C8H15NO2
MW: 157.213
Storage: 2-8 degree Celsius
SMILES: CC1[N@@](C1)C(=O)OC(C)(C)C
Catalog No.: 192585
Purity: 95%
MF: C8H15NO2
MW: 157.213
Storage: 2-8 degree Celsius
SMILES: CC1[N@@](C1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
(R)-tert-Butyl 2-methylaziridine-1-carboxylate; CAS No.: 129319-91-1; (R)-tert-Butyl 2-methylaziridine-1-carboxylate. PROPERTIES: (R)-tert-Butyl 2-methylaziridine-1-carboxylate is a colorless liquid with a molecular weight of 173.22 g/mol. It has a density of approximately 0.99 g/cm? and a boiling point around 105-110 C at 760 mmHg. This compound exhibits low water solubility but is miscible with organic solvents like ethyl acetate and tetrahydrofuran. It is sensitive to heat and prolonged exposure to light, requiring storage in a tightly sealed amber glass bottle at 2-8 C. Safety precautions include handling in a well-ventilated area, avoiding skin and eye contact as it may cause irritation. In case of spillage, absorb with inert material and dispose of according to hazardous waste regulations. The compound is a mild irritant and should not be heated above 40 C during processing. APPLICATIONS: (R)-tert-Butyl 2-methylaziridine-1-carboxylate is predominantly used as a chiral building block in asymmetric synthesis. Its aziridine ring provides a valuable three-membered ring structure for creating nitrogen-containing stereogenic centers in pharmaceuticals. For example, it is employed in the synthesis of certain antiviral drugs where the chiral aziridine motif enhances viral enzyme inhibition as described in antiviral chemistry research. Additionally, it serves as a precursor for creating chiral catalysts in organocatalysis, where the methyl substituent on the aziridine ring induces asymmetric induction during catalytic cycles, as reported in catalysis science literature. The compound is also utilized in polymer chemistry for creating chiral polypeptides with specific secondary structures, where the Boc protection allows for controlled polymerization and subsequent deprotection, as detailed in macromolecular chemistry studies. In agrochemical development, it acts as an intermediate for creating chiral herbicides where the aziridine ring facilitates soil adsorption and selective plant uptake, as outlined in pesticide chemistry publications.
Reviews
Write Your Own Review