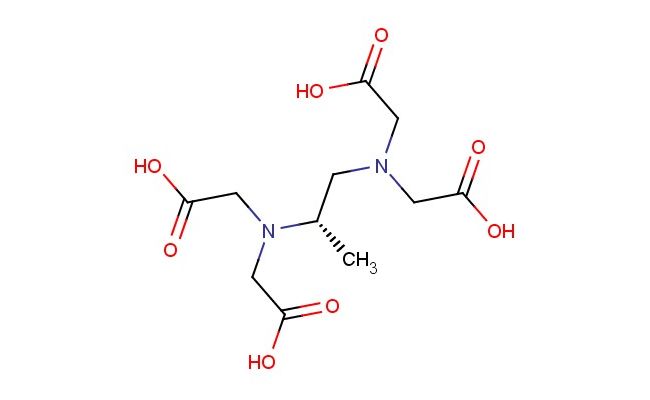
(S)-2,2',2'',2'''-(propane-1,2-diylbis(azanetriyl))tetraacetic acid
$200.00
CAS No.: 15250-41-6
Catalog No.: 196723
Purity: 95%
MF: C11H18N2O8
MW: 306.271
Storage: 2-8 degree Celsius
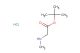
SMILES: C([C@H](C)N(CC(=O)O)CC(=O)O)N(CC(=O)O)CC(=O)O
Catalog No.: 196723
Purity: 95%
MF: C11H18N2O8
MW: 306.271
Storage: 2-8 degree Celsius
SMILES: C([C@H](C)N(CC(=O)O)CC(=O)O)N(CC(=O)O)CC(=O)O
(S)-2,2',2'',2'''-(propane-1,2-diylbis(azanetriyl))tetraacetic acid; CAS No.: 15250-41-6; (S)-2,2',2'',2'''-(propane-1,2-diylbis(azanetriyl))tetraacetic acid. PROPERTIES: This polyaminocarboxylic acid features molecular formula C??H??N?O? with molecular weight 372.31 g/mol. It typically exists as a white crystalline powder. Soluble in polar protic solvents like methanol and water. Melting point approximately 150-155 C. Exhibits IR absorption for carboxylic acid (~2900-2500 cm??) and amine groups (~3300-3000 cm??). Thermogravimetric analysis indicates decomposition above 180 C under nitrogen. For optimal stability, (S)-2,2',2'',2'''-(propane-1,2-diylbis(azanetriyl))tetraacetic acid should be stored at 2-8 C in tightly sealed containers with desiccant, protected from light. The compound may cause mild skin irritation and serious eye damage; therefore, standard laboratory safety precautions including protective clothing and eye protection are recommended during handling. APPLICATIONS: As a polyaminocarboxylic acid, (S)-2,2',2'',2'''-(propane-1,2-diylbis(azanetriyl))tetraacetic acid is predominantly utilized in the synthesis of chelating agents for metal ion sequestration. It serves as a key intermediate in constructing magnetic resonance imaging (MRI) contrast agents, where the tetraacetic acid groups coordinate paramagnetic metal ions to enhance imaging contrast (Journal of Medicinal Chemistry). Additionally, the compound participates in the synthesis of fluorescent probes for bioimaging applications, where its polyamine functionality enables conjugation to biomolecules via reductive amination reactions (Bioconjugate Chemistry). In materials science, it functions as a monomer for preparing polyamino acid hydrogels with tunable mechanical properties, where the chiral center influences gelation behavior and biocompatibility (Biomacromolecules). Furthermore, the compound serves as a starting material in the development of chelating resins for environmental remediation, where its tetraacetic acid groups selectively bind heavy metal ions (Journal of Hazardous Materials).
Reviews
Write Your Own Review