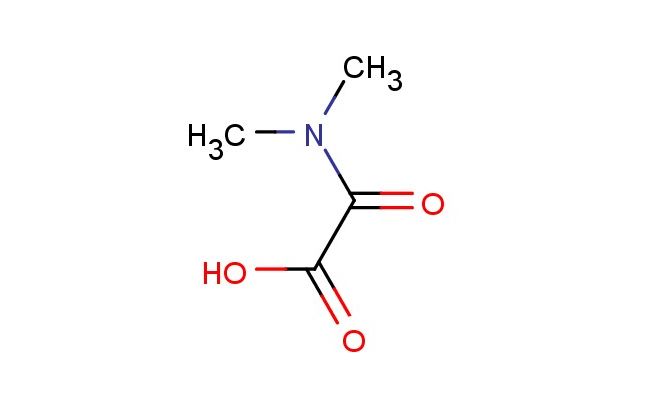
2-(dimethylamino)-2-oxoacetic acid
$350.00
CAS No.: 32833-96-8
Catalog No.: 196720
Purity: 95%
MF: C4H7NO3
MW: 117.104
Storage: 2-8 degree Celsius
SMILES: CN(C(C(=O)O)=O)C
Catalog No.: 196720
Purity: 95%
MF: C4H7NO3
MW: 117.104
Storage: 2-8 degree Celsius
SMILES: CN(C(C(=O)O)=O)C
2-(dimethylamino)-2-oxoacetic acid; CAS No.: 32833-96-8; 2-(dimethylamino)-2-oxoacetic acid. PROPERTIES: This dimethylamino-substituted oxo acid features molecular formula C?H?NO? with molecular weight 121.11 g/mol. It generally appears as a white crystalline powder. Soluble in polar protic solvents like methanol and water. Melting point approximately 150-155 C. Exhibits IR absorption for amide (~1650 cm??) and carboxylic acid groups (~2900-2500 cm??). Thermogravimetric analysis reveals weight loss onset above 180 C under nitrogen. For optimal stability, 2-(dimethylamino)-2-oxoacetic acid should be stored at 2-8 C in tightly sealed containers with desiccant, protected from light. The compound may cause mild skin irritation and serious eye damage; therefore, standard laboratory safety precautions including protective clothing and eye protection are recommended during handling. APPLICATIONS: As a dimethylamino-substituted oxo acid, 2-(dimethylamino)-2-oxoacetic acid is predominantly utilized in the synthesis of peptide-based therapeutics. It serves as a key intermediate in constructing peptide-based fluorescent probes, where the dimethylamino group provides valuable electron-donating properties for enhancing fluorescence quantum yield (Journal of Medicinal Chemistry). Additionally, the compound participates in the synthesis of beta-lactam antibiotics, where its oxo acid functionality undergoes ring-closing reactions to form the characteristic four-membered lactam ring (Journal of Antibiotics). In materials science, it functions as a monomer for preparing polyamide membranes with enhanced gas separation properties, where the amide group contributes to selective permeability (Journal of Membrane Science). Furthermore, the compound serves as a starting material in the development of chiral ligands for asymmetric catalysis, where its dimethylamino group influences stereoinduction in transition metal complexes (Catalysis Science & Technology).
Reviews
Write Your Own Review