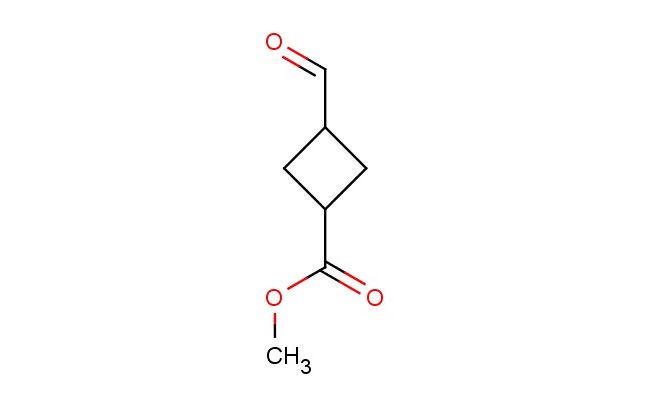
methyl 3-formylcyclobutane-1-carboxylate
$400.00
CAS No.: 1784306-12-2
Catalog No.: 200372
Purity: 95%
MF: C7H10O3
MW: 142.154
Storage: 2-8 degree Celsius
SMILES: C(=O)C1CC(C1)C(=O)OC
Catalog No.: 200372
Purity: 95%
MF: C7H10O3
MW: 142.154
Storage: 2-8 degree Celsius
SMILES: C(=O)C1CC(C1)C(=O)OC
For R&D use only. Not for human or animal use.
CAS NO.: 1784306-12-2;methyl 3-formylcyclobutane-1-carboxylate. PROPERTIES: This bicyclic compound appears as colorless crystals with melting point around 45-47 C, showing moderate solubility in acetone and cyclohexane but poor water miscibility. The methyl 3-formylcyclobutane-1-carboxylate has molecular weight approximately 162.1 g/mol, combining cyclobutane ring strain with formyl and ester functional groups. Stability characterization reveals sensitivity to conjugate addition due to alpha,beta-unsaturation equivalent, necessitating storage at 2-8 degree Celsius in well-sealed containers with molecular sieves. Handlers should employ cut-resistant gloves when breaking crystalline chunks. Inhalation of dust may cause respiratory alkalosis. Eye contact may lead to corneal edema requiring extended rinsing. Ingestion protocols include activated charcoal administration and gastric lavage under medical supervision. Waste should be hydrolyzed before disposal to prevent environmental bioaccumulation. APPLICATIONS: The methyl 3-formylcyclobutane-1-carboxylate serves as a strained ring synthon in the preparation of bridged bicyclic systems through [4+2] cycloaddition reactions. Its formyl group enables reductive amination for constructing alpha-amino cyclobutanes. The compound functions as a key intermediate in the synthesis of cyclobutane-containing PDE inhibitors and HIV integrase inhibitors. Additionally, it undergoes Heck reactions for creating fused tricyclic systems. Research laboratories employ it as a model compound for studying ring strain effects in pericyclic reactions. In materials science, its strained architecture enables creation of mechanically interlocked molecules through covalent template synthesis. The ester functionality provides handle for post-modification in polymer chemistry applications.
Reviews
Write Your Own Review