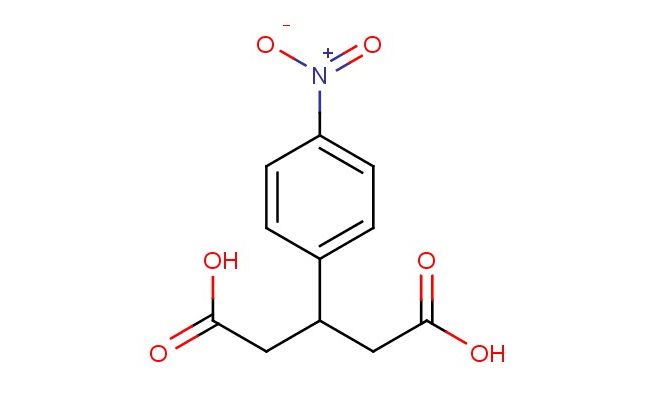
3-(4-nitrophenyl)pentanedioic acid
$200.00
CAS No.: 92289-14-0
Catalog No.: WLZ1218
Purity: 95%
MF: C11H11NO6
MW: 253.21
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C1=CC=C(C=C1)C(CC(=O)O)CC(=O)O
Catalog No.: WLZ1218
Purity: 95%
MF: C11H11NO6
MW: 253.21
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C1=CC=C(C=C1)C(CC(=O)O)CC(=O)O
For R&D use only. Not for human or animal use.
CAS NO.: 92289-14-0; 3-(4-nitrophenyl)pentanedioic acid. PROPERTIES: This aromatic dicarboxylic acid features a nitro-substituted phenyl group connected to a four-carbon alkane chain bearing carboxylic acid groups at both ends, creating a molecule with potential applications in chemical synthesis and analytical chemistry. The 3-(4-nitrophenyl)pentanedioic acid typically appears as a white crystalline powder with moderate aqueous solubility that increases with pH adjustment. Its molecular structure includes a nitro group that imparts electron-withdrawing character to the phenyl ring, influencing the acid dissociation behavior of the carboxylic acid groups. For optimal stability and to prevent degradation, this compound should be stored at 2-8 degree Celsius in a tightly sealed container away from moisture and direct light. When handling, appropriate safety measures including nitrile gloves and safety goggles are essential. This compound is hygroscopic and may form salts upon exposure to atmospheric moisture. In case of accidental spillage, clean the area with a damp cloth and dispose of materials according to local regulations. APPLICATIONS: The 3-(4-nitrophenyl)pentanedioic acid serves as a valuable intermediate in the synthesis of dyes, chelating agents, and pH indicators. The nitrophenyl group provides a chromophoric moiety that can be detected spectrophotometrically, making this compound useful in analytical chemistry applications. In medicinal chemistry, this acid functions as a building block for creating bioconjugates where the dicarboxylic acid groups can form amide bonds with amino-containing biomolecules. Additionally, the molecule finds utility in materials science as a cross-linking agent for polymers, leveraging the reactive carboxylic acid functionalities to create network structures. Researchers utilizing this compound benefit from its functional group versatility, enabling the development of diverse applications ranging from analytical reagents to functional materials.
Reviews
Write Your Own Review



![3-chloro-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/w/l/wlz1219_1.jpg)