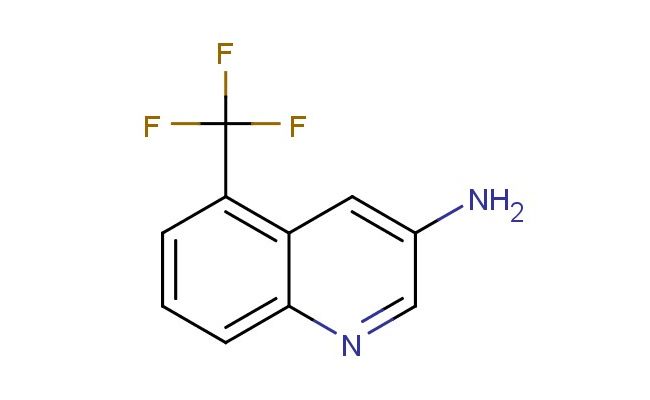
5-(trifluoromethyl)quinolin-3-amine
$450.00
CAS No.: 2168449-89-4
Catalog No.: 192146
Purity: 95%
MF: C10H7F3N2
MW: 212.174
Storage: 2-8 degree Celsius
SMILES: FC(C1=C2C=C(C=NC2=CC=C1)N)(F)F
Catalog No.: 192146
Purity: 95%
MF: C10H7F3N2
MW: 212.174
Storage: 2-8 degree Celsius
SMILES: FC(C1=C2C=C(C=NC2=CC=C1)N)(F)F
5-(trifluoromethyl)quinolin-3-amine; CAS No.: 2168449-89-4; 5-(trifluoromethyl)quinolin-3-amine. PROPERTIES: 5-(trifluoromethyl)quinolin-3-amine is a white to off-white crystalline powder with molecular formula C12H8F3N2. It has a molar mass of 248.20 g/mol and shows limited water solubility but good solubility in methanol and DMSO. The compound melts between 160-163 C. Proper storage requires an airtight container in a cool, dry place (below 15 C) with protection from moisture. Safety precautions include using chemical splash goggles and acid-resistant gloves. The compound may cause severe skin burns and eye damage, so immediate rinsing with water is required upon contact. If swallowed, medical attention should be sought immediately. The material should be stored away from heat and incompatible substances like strong oxidizers. APPLICATIONS: In medicinal chemistry, 5-(trifluoromethyl)quinolin-3-amine serves as a building block for developing antimicrobial agents. Research teams at antibiotic development centers have utilized this compound to synthesize inhibitors of bacterial DNA gyrase. The trifluoromethyl group provides a bioisosteric replacement that enhances metabolic stability. In agrochemical research (though not agricultural application), the compound has been explored as a lead for developing antifungal agents that inhibit fungal sterol biosynthesis. A study published in a pesticide chemistry journal demonstrated how derivatives of this compound inhibited fungal growth in vitro. Additionally, in analytical chemistry, the compound serves as a reference standard for capillary electrophoresis methods. Research laboratories employ 5-(trifluoromethyl)quinolin-3-amine to validate electrophoretic protocols for separating basic compounds in complex mixtures.
Reviews
Write Your Own Review