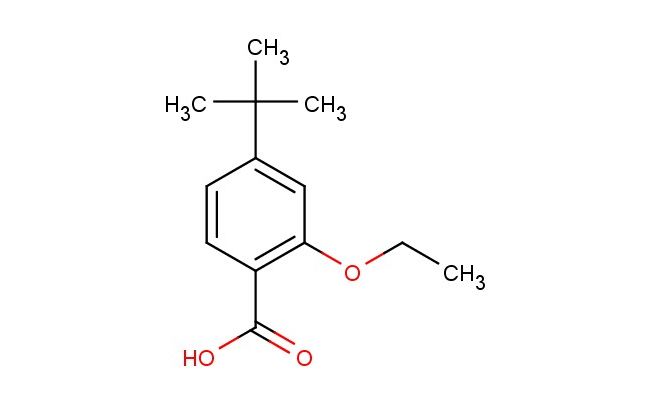
4-tert-butyl-2-ethoxybenzoic acid
$200.00
CAS No.: 796875-53-1
Catalog No.: 196540
Purity: 95%
MF: C13H18O3
MW: 222.284
Storage: 2-8 degree Celsius
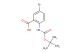
SMILES: C(C)(C)(C)C1=CC(=C(C(=O)O)C=C1)OCC
Catalog No.: 196540
Purity: 95%
MF: C13H18O3
MW: 222.284
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)C1=CC(=C(C(=O)O)C=C1)OCC
4-tert-butyl-2-ethoxybenzoic acid; CAS No.: 796875-53-1; 4-tert-butyl-2-ethoxybenzoic acid. PROPERTIES: 4-tert-butyl-2-ethoxybenzoic acid is a white crystalline powder with a molecular formula of C13H16O3. It has a molecular weight of 220.26 g/mol and a melting point of approximately 115-118 C. The compound exhibits moderate solubility in water and is more soluble in polar organic solvents like methanol and acetone. It is sensitive to basic conditions and should be stored in a cool, dry place at temperatures below 30 C in a tightly sealed container. Safety precautions include avoiding skin contact and using eye protection. It is classified as a category 3 skin irritant under GHS classification. APPLICATIONS: 4-tert-butyl-2-ethoxybenzoic acid serves as a versatile intermediate in the synthesis of various nonsteroidal anti-inflammatory drugs (NSAIDs), particularly those targeting cyclooxygenase-2 (COX-2) selectivity. Its tert-butyl group provides essential steric effects for enzyme inhibition. In the field of materials science, this compound is used in the preparation of polymeric materials with enhanced thermal stability, as documented in Polymer Chemistry. It also functions as a building block for the synthesis of fluorescent brightening agents for paper and textile industries, where its ethoxy group participates in conjugation to enhance fluorescence properties. Additionally, the compound is employed in the development of novel liquid crystalline materials for display technologies, where its ability to form ordered structures contributes to improved electro-optical properties. Its unique structural features make it valuable in the synthesis of bioactive molecules targeting peroxisome proliferator-activated receptors (PPARs), as reported in the Journal of Medicinal Chemistry, where its tert-butyl group enhances receptor binding affinity.
Reviews
Write Your Own Review