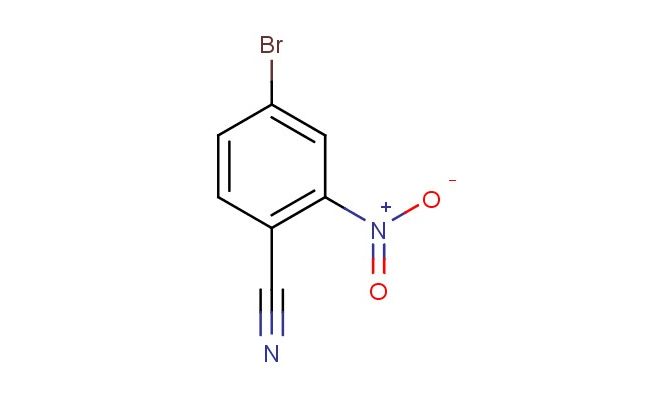
4-bromo-2-nitrobenzonitrile
$400.00
CAS No.: 79603-03-5
Catalog No.: 196538
Purity: 95%
MF: C7H3BrN2O2
MW: 227.017
Storage: 2-8 degree Celsius
SMILES: BrC1=CC(=C(C#N)C=C1)[N+](=O)[O-]
Catalog No.: 196538
Purity: 95%
MF: C7H3BrN2O2
MW: 227.017
Storage: 2-8 degree Celsius
SMILES: BrC1=CC(=C(C#N)C=C1)[N+](=O)[O-]
4-bromo-2-nitrobenzonitrile; CAS No.: 79603-03-5; 4-bromo-2-nitrobenzonitrile. PROPERTIES: 4-bromo-2-nitrobenzonitrile is a pale yellow crystalline solid with a molecular formula of C7H3BrN2O2. It has a molecular weight of 227.02 g/mol and a melting point of approximately 78-82 C. The compound exhibits low water solubility but is soluble in polar aprotic solvents like DMSO and DMF. It is sensitive to reducing conditions and should be stored below 10 C in a tightly sealed container, protected from light and moisture. Safety precautions include using explosion-proof ventilation and avoiding skin contact. It is classified as an oxidizing agent (Oxidation category 2) and a category 2 skin irritant. APPLICATIONS: 4-bromo-2-nitrobenzonitrile serves as a key intermediate in the synthesis of various azo dyes with improved colorfastness properties. Its nitro group provides essential electron-withdrawing effects for dye stability. In the field of medicinal chemistry, this compound is used in the preparation of antimalarial agents where its nitroaromatic group undergoes bioreduction to generate toxic metabolites in Plasmodium species. It also functions as a building block for the synthesis of fluorescent probes for detecting nitroreductase activity in tumor microenvironments, as reported in Bioconjugate Chemistry. Additionally, the compound is employed in the development of novel chemosensors for explosive detection, where its electron-deficient aromatic system interacts with nitroaromatic explosives to produce visible color changes. Its unique electronic properties make it valuable in the synthesis of organic semiconductors for field-effect transistor applications, as described in Advanced Materials, where its ability to form ordered molecular assemblies enhances charge transport properties.
Reviews
Write Your Own Review