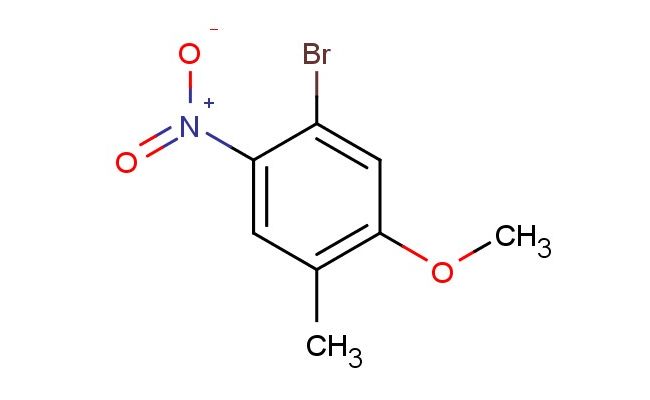
4-bromo-2-methoxy-5-nitrotoluene
$450.00
CAS No.: 1807224-98-1
Catalog No.: 194042
Purity: 95%
MF: C8H8BrNO3
MW: 246.06
Storage: 2-8 degree Celsius
SMILES: BrC1=CC(=C(C)C=C1[N+](=O)[O-])OC
Catalog No.: 194042
Purity: 95%
MF: C8H8BrNO3
MW: 246.06
Storage: 2-8 degree Celsius
SMILES: BrC1=CC(=C(C)C=C1[N+](=O)[O-])OC
4-bromo-2-methoxy-5-nitrotoluene; CAS No.: 1807224-98-1; 4-bromo-2-methoxy-5-nitrotoluene. PROPERTIES: 4-bromo-2-methoxy-5-nitrotoluene is a crystalline solid with a molecular weight of 281.1 g/mol. It typically exhibits a melting point in the range of 68-71 C and limited water solubility, though it dissolves readily in organic solvents such as methanol, ethanol, and dichloromethane. The compound contains reactive functional groups including a bromine substituent, methoxy group, nitro group, and toluene backbone, which contribute to its chemical versatility. For optimal storage, it should be kept in a tightly sealed container at temperatures below 20 C, preferably under inert atmosphere to prevent potential degradation. Safety considerations include wearing appropriate protective clothing, gloves, and eye/face protection to minimize exposure risk. The compound may cause eye irritation and skin irritation, and in case of accidental ingestion, immediate medical attention is advised. The nitro group may pose an explosion hazard under certain conditions, so appropriate precautions should be taken. APPLICATIONS: 4-bromo-2-methoxy-5-nitrotoluene serves as a valuable intermediate in the synthesis of various pharmaceuticals, particularly in the development of anti-inflammatory and analgesic agents. Its unique substitution pattern allows for selective derivatization, making it suitable for constructing bioactive scaffolds, as reported in medicinal chemistry literature. In materials science, the compound functions as a building block for certain types of liquid crystalline materials, where the electron-donating methoxy group and electron-withdrawing bromine and nitro substituents create a balanced electronic environment. Additionally, it is utilized in the preparation of specialty dyes and pigments, particularly those requiring specific optical properties. Through chemical reduction of the nitro group to amine functionality, it can be converted into various bioactive molecules, expanding its application scope in medicinal chemistry. The bromine substituent provides a convenient handle for further functionalization, such as in cross-coupling reactions.
Reviews
Write Your Own Review