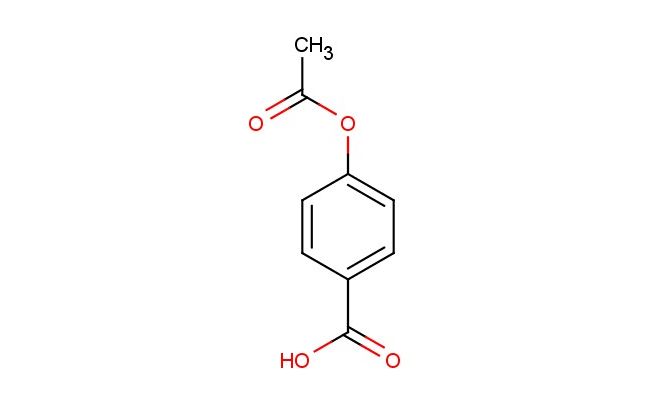
4-Acetoxybenzoic acid
$300.00
CAS No.: 2345-34-8
Catalog No.: WLZ1209
Purity: 95%
MF: C9H8O4
MW: 180.159
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)OC1=CC=C(C(=O)O)C=C1
Catalog No.: WLZ1209
Purity: 95%
MF: C9H8O4
MW: 180.159
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)OC1=CC=C(C(=O)O)C=C1
For R&D use only. Not for human or animal use.
CAS NO.: 2345-34-8; 4-Acetoxybenzoic acid. PROPERTIES: This aromatic carboxylic acid features an acetoxy group para to the carboxylic acid functionality on a benzene ring, creating a molecule with potential applications in organic synthesis and pharmaceutical research. The 4-acetoxybenzoic acid typically appears as a white crystalline powder with moderate aqueous solubility that increases with pH adjustment. Its molecular structure includes an ester-like acetoxy group that can undergo hydrolysis under acidic or basic conditions to release salicylic acid derivatives. For optimal stability and to prevent hydrolysis, this compound should be stored at 2-8 degree Celsius in a tightly sealed container under anhydrous conditions. When handling, appropriate safety measures including nitrile gloves and safety goggles are recommended. This compound is sensitive to moisture and may form salts upon exposure to atmospheric moisture. In case of accidental ingestion, rinse mouth thoroughly and seek immediate medical attention. APPLICATIONS: The 4-acetoxybenzoic acid serves as a valuable intermediate in the synthesis of pharmaceuticals, particularly nonsteroidal anti-inflammatory drugs (NSAIDs) and antimicrobial agents. The acetoxy group provides a protected hydroxyl functionality that can be selectively deprotected during synthesis. In medicinal chemistry, this compound functions as a building block for creating prodrugs designed to improve the bioavailability of aromatic acids. Additionally, the molecule finds utility in organic synthesis as a starting material for preparing substituted benzene derivatives with diverse biological activities. Researchers utilizing this compound benefit from its functional group compatibility, enabling the development of therapeutic agents targeting various disease pathways including inflammation and infection.
Reviews
Write Your Own Review