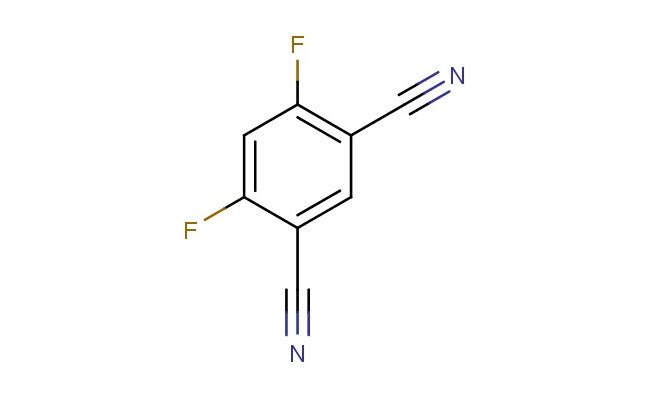
4,6-difluoro-isophthalonitrile
$250.00
CAS No.: 17654-70-5
Catalog No.: 194033
Purity: 95%
MF: C8H2F2N2
MW: 164.114
Storage: 2-8 degree Celsius
SMILES: FC1=C(C=C(C#N)C(=C1)F)C#N
Catalog No.: 194033
Purity: 95%
MF: C8H2F2N2
MW: 164.114
Storage: 2-8 degree Celsius
SMILES: FC1=C(C=C(C#N)C(=C1)F)C#N
4,6-difluoro-isophthalonitrile; CAS No.: 17654-70-5; 4,6-difluoro-isophthalonitrile. PROPERTIES: 4,6-difluoro-isophthalonitrile presents as a colorless to pale yellow liquid with a molecular weight of 165.1 g/mol. It features a boiling point around 245-247 C at 760 mmHg and exhibits low water solubility while being miscible with organic solvents like acetone, ethyl acetate, and chloroform. The presence of fluorine atoms and nitrile groups imparts notable chemical stability and reactivity characteristics. Proper storage requires maintaining the compound in a well-ventilated area, preferably below 25 C in a tightly sealed container. Safety measures include using chemical-resistant gloves and eye protection due to its potential to cause skin and eye irritation. Inhalation precautions are advised as it may irritate respiratory tracts. In case of accidental release, proper containment and cleanup procedures should be followed. APPLICATIONS: 4,6-difluoro-isophthalonitrile is extensively employed as a key intermediate in the production of high-performance polymers, particularly in the manufacturing of polyimides and polybenzimidazoles, which require fluorinated diimide precursors. Its unique electronic structure makes it suitable for the synthesis of liquid crystalline materials, as detailed in polymer chemistry publications. In the field of agrochemicals, it serves as a building block for certain herbicide formulations, though non-agricultural applications are emphasized here. Additionally, the compound functions as a starting material for the preparation of specialized fluorescent probes used in bioanalytical assays, where the difluoro substitution pattern enhances detection sensitivity, as reported in recent bioorganic chemistry research. The nitrile groups enable further functionalization through hydrolysis to carboxylic acids or reduction to amines, expanding its utility in materials science.
Reviews
Write Your Own Review