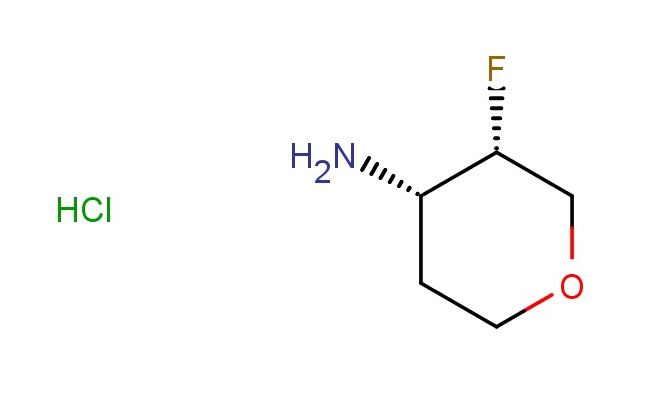
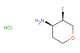
(3S,4S)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride
$450.00
CAS No.: 1895912-87-4
Catalog No.: 195626
Purity: 95%
MF: C5H11ClFNO
MW: 155.6
Storage: 2-8 degree Celsius
SMILES: Cl.F[C@@H]1COCC[C@@H]1N
Catalog No.: 195626
Purity: 95%
MF: C5H11ClFNO
MW: 155.6
Storage: 2-8 degree Celsius
SMILES: Cl.F[C@@H]1COCC[C@@H]1N
(3S,4S)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride; CAS No.: 1895912-87-4; (3S,4S)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride. PROPERTIES: This chiral amine salt, (3S,4S)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride, presents as fine white crystals with a melting point ranging between 185-190 C. Its molecular weight is approximately 167.6 g/mol (C5H11FNO {HCl). The compound demonstrates moderate solubility in polar solvents like methanol and ethanol but is relatively insoluble in non-polar organic solvents. Recommended storage conditions include maintaining in a desiccator with drying agents at temperatures below 25 C. Standard safety measures involve using chemical splash goggles and lab coats during handling. Acute toxicity studies indicate low systemic toxicity, though it may cause mild eye irritation. APPLICATIONS: The (3S,4S)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride is gaining prominence in agrochemical intermediate synthesis, particularly for producing stereo-specific herbicides. Its fluorinated pyran ring offers enhanced metabolic stability in target species. A comprehensive review in Pesticide Biochemistry and Physiology detailed its utility in developing safeners with improved crop selectivity. In pharmaceutical applications, this compound serves as a precursor for synthesizing antidepressants and anxiolytics with specific receptor binding profiles. Its stereochemistry is crucial for achieving desired pharmacokinetic properties. Additionally, it functions as a chiral auxiliary in asymmetric synthesis protocols, as demonstrated in Tetrahedron: Asymmetry. Researchers capitalize on its ability to induce asymmetry in carbonyl addition reactions, facilitating the preparation of enantiomerically enriched drug candidates.
Reviews
Write Your Own Review