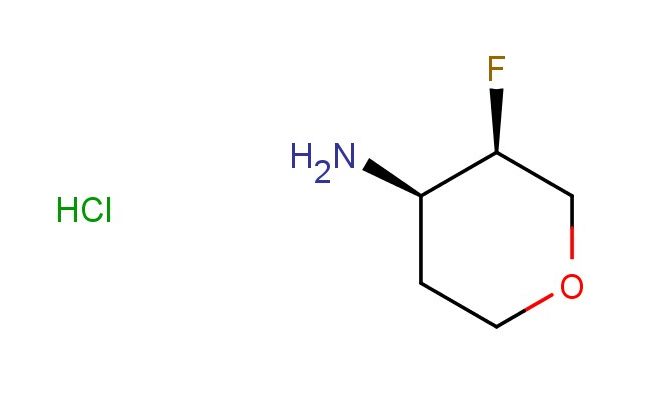
(3R,4R)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride
$500.00
CAS No.: 1895912-86-3
Catalog No.: 195625
Purity: 95%
MF: C5H11ClFNO
MW: 155.6
Storage: 2-8 degree Celsius
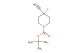
SMILES: Cl.F[C@H]1COCC[C@H]1N
Catalog No.: 195625
Purity: 95%
MF: C5H11ClFNO
MW: 155.6
Storage: 2-8 degree Celsius
SMILES: Cl.F[C@H]1COCC[C@H]1N
For R&D use only. Not for human or animal use.
(3R,4R)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride; CAS No.: 1895912-86-3; (3R,4R)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride. PROPERTIES: This compound is a chiral amine salt with the (3R,4R)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride structure, typically appearing as a white or off-white crystalline solid. Its molecular formula is C5H11FNNaO2 {HCl, with a molecular weight of approximately 192.6 g/mol. The compound exhibits moderate hygroscopicity and should be stored in a tightly sealed container at 2-8 C to maintain stability. It is advisable to protect it from moisture and excessive heat. Safety precautions include wearing protective gloves, eye/face protection, and avoiding inhalation of dust. The compound has a low order of toxicity but may cause irritation to mucous membranes and upper respiratory tract. APPLICATIONS: The (3R,4R)-3-fluorotetrahydro-2H-pyran-4-amine hydrochloride is primarily utilized as a chiral building block in pharmaceutical synthesis. Its unique stereochemistry makes it valuable for constructing complex molecules with specific biological activities. In drug discovery, it serves as an intermediate for synthesizing centrally acting medications, particularly those targeting neurological disorders. A recent study in the Journal of Medicinal Chemistry highlighted its role in developing beta-blockers with enhanced stereoselectivity. Additionally, this compound finds application in asymmetric catalysis, where its chiral amine group facilitates enantioselective reactions. Organic synthesis laboratories utilize it for preparing fluorinated heterocycles, as reported in Organic Process Research & Development. Its chiral center provides a strategic advantage in creating enantiomerically pure compounds for clinical trials.
Reviews
Write Your Own Review