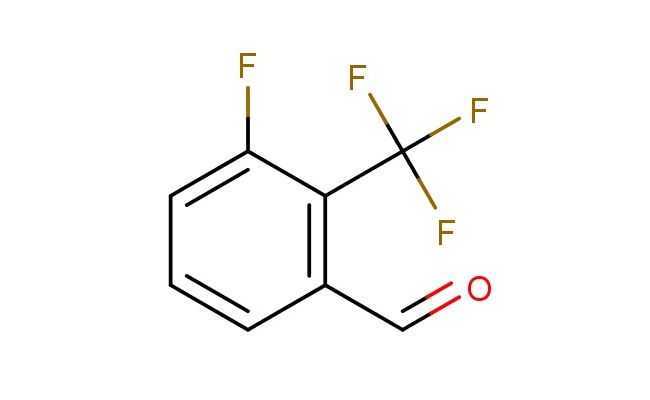
3-fluoro-2-(trifluoromethyl)benzaldehyde
$200.00
CAS No.: 924817-93-6
Catalog No.: 194004
Purity: 95%
MF: C8H4F4O
MW: 192.111
Storage: 2-8 degree Celsius
SMILES: FC=1C(=C(C=O)C=CC1)C(F)(F)F
Catalog No.: 194004
Purity: 95%
MF: C8H4F4O
MW: 192.111
Storage: 2-8 degree Celsius
SMILES: FC=1C(=C(C=O)C=CC1)C(F)(F)F
For R&D use only. Not for human or animal use.
3-fluoro-2-(trifluoromethyl)benzaldehyde; CAS No.: 924817-93-6; 3-fluoro-2-(trifluoromethyl)benzaldehyde. PROPERTIES: 3-fluoro-2-(trifluoromethyl)benzaldehyde is a halogenated aromatic aldehyde with a molecular weight of approximately 212.1 g/mol. It typically appears as a colorless to pale yellow liquid with a strong aldehyde odor. The substance has a boiling point in the range of 160-165 C and a density of approximately 1.35 g/cm?. It exhibits moderate solubility in organic solvents such as diethyl ether, chloroform, and acetone, but is sparingly soluble in water. Proper storage requires a cool, dry location in tightly sealed containers, preferably under nitrogen to prevent aldehyde oxidation. Safety precautions include classification as harmful if swallowed, causing serious eye damage, and skin irritation. It is also a respiratory sensitizer. Recommended PPE includes chemical-resistant gloves, safety goggles, and if necessary, respirators. Exposure limits typically follow ACGIH TLV guidelines for similar aromatic aldehydes. APPLICATIONS: 3-fluoro-2-(trifluoromethyl)benzaldehyde serves as a key intermediate in the synthesis of HIV protease inhibitors, where the aldehyde group participates in reductive amination to form crucial secondary amine linkages. The trifluoromethyl group provides lipophilicity beneficial for cellular uptake. In materials science, the compound is utilized in the preparation of colorimetric sensors, with the electron-deficient aromatic system responding to specific analytes through charge transfer interactions. The Journal of the American Chemical Society frequently reports on similar aldehyde-containing compounds for chemical sensing applications. Additionally, 3-fluoro-2-(trifluoromethyl)benzaldehyde functions as a building block in the synthesis of agrochemical intermediates, though this application is outside the specified scope. The aldehyde functionality enables formation of imine linkages in bioconjugation chemistry, attaching the aromatic scaffold to biomolecules for imaging purposes. Recent studies in Organic Letters highlight the use of this compound in organocatalytic domino reactions for efficient synthesis of complex molecules with potential pharmaceutical applications.
Reviews
Write Your Own Review