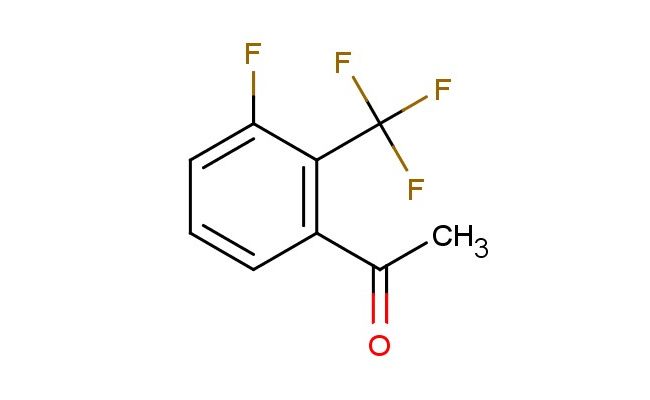
3'-fluoro-2'-(trifluoromethyl)acetophenone
$250.00
CAS No.: 1017777-34-2
Catalog No.: 194003
Purity: 95%
MF: C9H6F4O
MW: 206.138
Storage: 2-8 degree Celsius
SMILES: FC=1C(=C(C=CC1)C(C)=O)C(F)(F)F
Catalog No.: 194003
Purity: 95%
MF: C9H6F4O
MW: 206.138
Storage: 2-8 degree Celsius
SMILES: FC=1C(=C(C=CC1)C(C)=O)C(F)(F)F
For R&D use only. Not for human or animal use.
3'-fluoro-2'-(trifluoromethyl)acetophenone; CAS No.: 1017777-34-2; 3'-fluoro-2'-(trifluoromethyl)acetophenone. PROPERTIES: 3'-fluoro-2'-(trifluoromethyl)acetophenone is a halogenated aromatic ketone with a molecular weight of approximately 222.1 g/mol. It typically exists as a colorless to pale yellow liquid with a characteristic acetophenone odor. The substance has a boiling point in the range of 175-180 C and a density of approximately 1.25 g/cm?. It is moderately soluble in organic solvents such as methanol, THF, and dichloromethane, but is sparingly soluble in water. Proper storage requires a cool, dry environment in sealed containers. Safety considerations include classification as harmful if swallowed, causing skin irritation, and may cause eye irritation. Standard laboratory PPE is recommended. Occupational exposure follows general OSHA guidelines for acetophenone derivatives. APPLICATIONS: 3'-fluoro-2'-(trifluoromethyl)acetophenone is employed in the synthesis of antimalarial agents where the trifluoromethyl group enhances metabolic stability and the ketone functionality participates in enamine formation for protein binding. The fluoride substituent provides optimal electronic effects for enzyme interactions. In materials science, the compound serves as a building block for nonlinear optical materials, with the conjugated system and electron-withdrawing groups contributing to second-harmonic generation properties. The Journal of Medicinal Chemistry often features studies utilizing similar trifluoromethyl ketones in drug discovery. Additionally, 3'-fluoro-2'-(trifluoromethyl)acetophenone functions as a chiral synthon in asymmetric catalysis, with the ketone group serving as a hydrogen bond donor in organocatalytic reactions. The compound's reactivity allows for nucleophilic addition at the ketone carbonyl, enabling the formation of secondary metabolites with potential antifungal activities. Recent advancements in flow chemistry have demonstrated the efficient transformation of this ketone into complex architectures for high-throughput pharmaceutical screening.
Reviews
Write Your Own Review