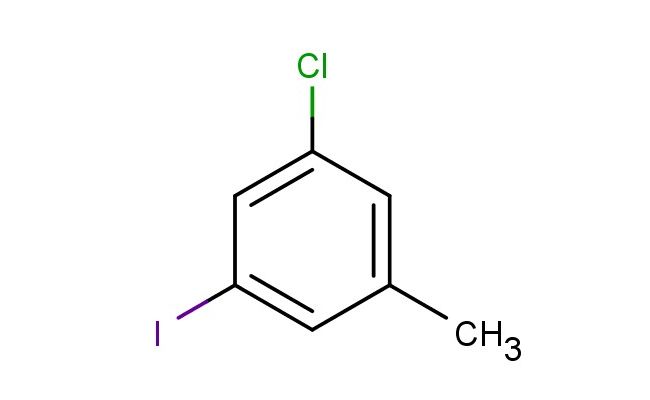
3-chloro-5-iodotoluene
$300.00
CAS No.: 116632-43-0
Catalog No.: 193999
Purity: 95%
MF: C7H6ClI
MW: 252.482
Storage: 2-8 degree Celsius
SMILES: ClC=1C=C(C)C=C(C1)I
Catalog No.: 193999
Purity: 95%
MF: C7H6ClI
MW: 252.482
Storage: 2-8 degree Celsius
SMILES: ClC=1C=C(C)C=C(C1)I
3-chloro-5-iodotoluene; CAS No.: 116632-43-0; 3-chloro-5-iodotoluene. PROPERTIES: 3-chloro-5-iodotoluene is a dihalogenated aromatic hydrocarbon with a molecular weight of approximately 271.5 g/mol. It typically exists as colorless crystals with a faint aromatic odor. The substance has a melting point ranging from 32-35 C and a boiling point of approximately 220-225 C. It exhibits low solubility in water but is soluble in organic solvents such as dichloromethane, acetone, and ethyl acetate. The density is around 1.75 g/cm?, reflecting the significant iodine content. Proper storage requires a cool, dry environment in tightly sealed containers, preferably under an inert atmosphere to prevent potential oxidation. Safety considerations include classification as harmful if swallowed, causing skin irritation, and serious eye damage. It may also cause respiratory irritation. Recommended personal protective equipment includes NIOSH-approved respirators, chemical-resistant gloves, and safety goggles. Occupational exposure limits typically follow ACGIH Threshold Limit Values (TLV) for similar halogenated aromatics. APPLICATIONS: 3-chloro-5-iodotoluene serves as a valuable cross-coupling partner in Suzuki-Miyaura reactions due to its iodine handle, enabling the formation of biaryl structures important in pharmaceuticals and materials science. In medicinal chemistry, the compound is utilized in the synthesis of kinase inhibitors where the meta-chloro and para-iodo substituents provide optimal electronic effects for enzyme binding. The Journal of Organic Chemistry reports numerous examples of such couplings facilitating the preparation of complex molecules with desired stereochemical outcomes. Additionally, 3-chloro-5-iodotoluene functions as a precursor in the preparation of liquid crystalline materials, where the halogen substituents tune the mesomorphic properties. In materials science, derivatives of this compound have been employed in the development of organic semiconductors, where the halogen atoms influence charge transport characteristics. The compound is also used in the synthesis of certain agrochemicals, though this application is outside the specified scope. Recent advancements in transition metal-catalyzed cross-couplings have expanded the utility of 3-chloro-5-iodotoluene in accessing diverse aromatic architectures for optoelectronic applications.
Reviews
Write Your Own Review