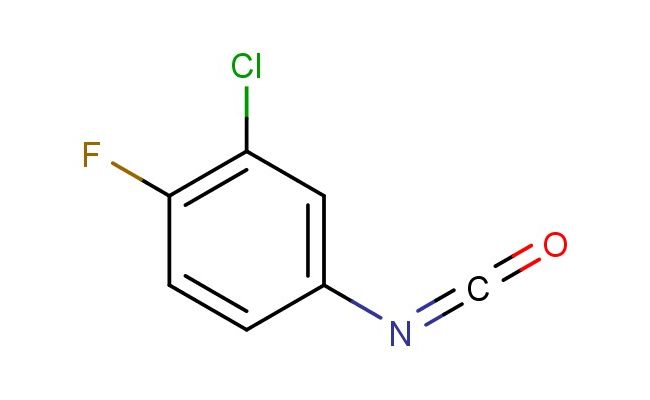
3-chloro-4-fluorophenyl isocyanate
$300.00
CAS No.: 50529-33-4
Catalog No.: 193998
Purity: 95%
MF: C7H3ClFNO
MW: 171.558
Storage: 2-8 degree Celsius
SMILES: ClC=1C=C(C=CC1F)N=C=O
Catalog No.: 193998
Purity: 95%
MF: C7H3ClFNO
MW: 171.558
Storage: 2-8 degree Celsius
SMILES: ClC=1C=C(C=CC1F)N=C=O
3-chloro-4-fluorophenyl isocyanate; CAS No.: 50529-33-4; 3-chloro-4-fluorophenyl isocyanate. PROPERTIES: 3-chloro-4-fluorophenyl isocyanate is a highly reactive aromatic isocyanate with a molecular weight of approximately 167.5 g/mol. It typically appears as a colorless to pale yellow liquid with a pungent odor characteristic of isocyanates. The substance is sensitive to moisture and hydrolyzes in the presence of water to form ureas and release carbon dioxide. It has a boiling point in the range of 120-130 C and a density of approximately 1.28 g/cm?. Proper storage requires a dry, inert atmosphere (nitrogen or argon) in sealed containers, preferably amber glass to protect from light. The substance is highly toxic, corrosive, and poses serious health hazards including severe skin burns, eye damage, and respiratory irritation. Safety measures include wearing fully enclosed chemical-resistant suits, self-contained breathing apparatus (SCBA), and nitrile gloves. Exposure limits are extremely strict, with OSHA PEL typically set below 0.02 ppm (ceiling). APPLICATIONS: 3-chloro-4-fluorophenyl isocyanate is primarily used in the production of polyurethane foams with specialized properties, particularly in applications requiring flame retardancy due to the halogenated nature of the isocyanate. In pharmaceutical synthesis, the compound serves as a building block for certain antiparasitic and antifungal agents, where the isocyanate group reacts with amines to form urea linkages critical for biological activity. The reagent is also employed in the preparation of specialty dyes and pigments, where the isocyanate functionality enables the formation of color bodies with enhanced stability. The reaction of 3-chloro-4-fluorophenyl isocyanate with alcohols and phenols produces carbamates that find use in the synthesis of agrochemical intermediates, though this application is outside the specified scope. Industrial coatings formulations occasionally incorporate derivatives of this isocyanate to improve chemical resistance and adhesion properties. The Polymer Chemistry journal frequently documents the utilization of halogenated aromatic isocyanates in developing novel polyurethane formulations.
Reviews
Write Your Own Review