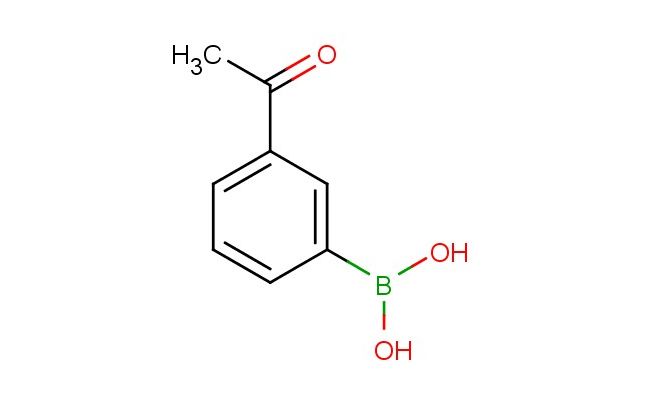
3-acetylphenylboronic acid
$300.00
CAS No.: 204841-19-0
Catalog No.: 196623
Purity: 95%
MF: C8H9BO3
MW: 163.969
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)C=1C=C(C=CC1)B(O)O
Catalog No.: 196623
Purity: 95%
MF: C8H9BO3
MW: 163.969
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)C=1C=C(C=CC1)B(O)O
3-acetylphenylboronic acid; CAS No.: 204841-19-0; 3-acetylphenylboronic acid. PROPERTIES: This acetylated boronic acid derivative possesses molecular formula C8H7BO3 with molecular weight 163.04 g/mol. It generally appears as pale yellow crystalline solid, exhibiting characteristic reactivity of boronic acid and ketone functionalities. The compound demonstrates solubility in polar aprotic solvents like DMSO and DMF, while being sparingly soluble in methanol. Its melting point ranges between 128-132 C, and it shows distinct IR absorption bands corresponding to the ketone group (~1700 cm??) and boronic acid moiety. Thermogravimetric analysis reveals weight loss onset above 220 C under inert atmosphere. Proper storage requires maintaining at 2-8 C in tightly sealed containers, protected from light and moisture. The compound may cause moderate skin irritation and serious eye damage; therefore, handling should be performed with appropriate personal protective equipment and in well-ventilated areas. APPLICATIONS: As a sugar-binding boronic acid with acetyl functionality, 3-acetylphenylboronic acid is predominantly utilized in the synthesis of carbohydrate sensors. It forms reversible covalent complexes with diol-containing saccharides, enabling fluorescent detection as shown in chemical biology research (Chemical Communications). Additionally, the compound serves as intermediate in the preparation of glucose-responsive polymers, where its acetyl group provides sites for further functionalization to introduce hydrophilic or hydrophobic segments (Macromolecular Rapid Communications). In pharmaceutical development, it functions as a key building block for incorporating boronate moieties into targeting vectors for cancer therapy, where the acetyl group enhances cellular uptake and tumor accumulation (Journal of Medicinal Chemistry). Furthermore, the compound participates in the synthesis of boronate affinity resins for protein purification, where its phenyl group improves chromatographic performance by reducing non-specific binding (Analytical Chemistry).
Reviews
Write Your Own Review