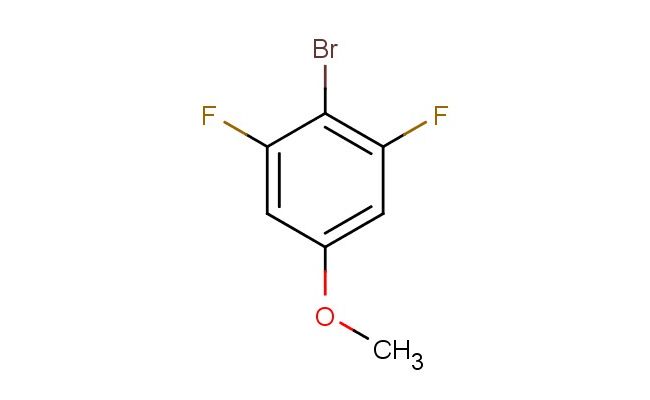
2-bromo-1,3-difluoro-5-methoxybenzene
$300.00
CAS No.: 202865-61-0
Catalog No.: 196640
Purity: 95%
MF: C7H5BrF2O
MW: 223.016
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=C(C=C1F)OC)F
Catalog No.: 196640
Purity: 95%
MF: C7H5BrF2O
MW: 223.016
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C=C(C=C1F)OC)F
2-bromo-1,3-difluoro-5-methoxybenzene; CAS No.: 202865-61-0; 2-bromo-1,3-difluoro-5-methoxybenzene. PROPERTIES: This bromo-difluoro-methoxy-substituted aromatic compound features molecular formula C7H5BrF2O with molecular weight 219.02 g/mol. It typically exists as colorless liquid, exhibiting characteristic reactivity of bromo, fluoro, and methoxy substituents. The compound demonstrates solubility in non-polar and slightly polar organic solvents like hexanes and dichloromethane, while being sparingly soluble in water. Its boiling point ranges between 130-135 C at 760 mmHg, and it exhibits IR absorption bands corresponding to the C-Br (~600-500 cm??), C-F (~1300-1100 cm??), and C-O (~1200-1000 cm??) stretches. Thermogravimetric analysis reveals decomposition onset above 150 C under nitrogen atmosphere. For optimal stability, 2-bromo-1,3-difluoro-5-methoxybenzene should be stored at 2-8 C in tightly sealed amber glass containers, protected from moisture and prolonged light exposure. As with brominated compounds, it may cause severe skin burns and eye damage; therefore, rigorous containment and personal protection measures are essential during manipulation. APPLICATIONS: The versatile halogenation pattern of 2-bromo-1,3-difluoro-5-methoxybenzene makes it valuable as a building block in pharmaceutical and agrochemical syntheses. It serves as key intermediate in the preparation of diverse aryl-containing compounds through sequential nucleophilic aromatic substitution and cross-coupling reactions, where the bromo, fluoro, and methoxy groups provide orthogonal reactivity as demonstrated in organic synthesis research (Journal of Fluorine Chemistry). Additionally, the compound participates in the synthesis of liquid crystal materials, where its bromodifluoromethoxy substitution pattern contributes to desired mesomorphic properties and thermal stability (Liquid Crystals). In materials science, it functions as monomer for preparing polyaryl ethers with enhanced flame retardancy, where the bromo and fluoro substituents provide valuable fire-resistant characteristics (Polymer International). Furthermore, the compound serves as starting material in the development of brominated solvents with specialized applications in chemical processes requiring specific polarity and solvation properties (Green Chemistry).
Reviews
Write Your Own Review