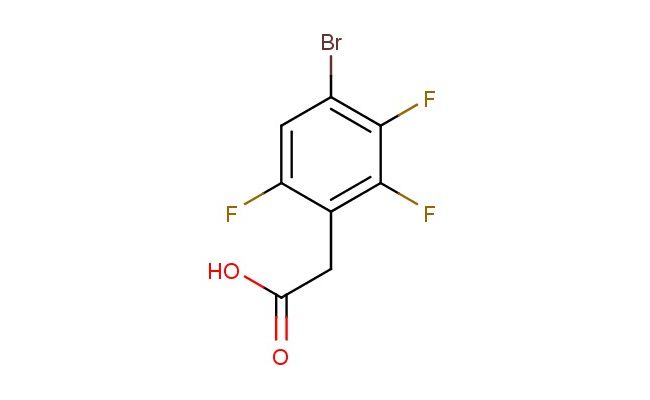
2-(4-bromo-2,3,6-trifluorophenyl)acetic acid
$200.00
CAS No.: 537033-59-3
Catalog No.: 197161
Purity: 95%
MF: C8H4BrF3O2
MW: 269.016
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C(=C(C(=C1)F)CC(=O)O)F)F
Catalog No.: 197161
Purity: 95%
MF: C8H4BrF3O2
MW: 269.016
Storage: 2-8 degree Celsius
SMILES: BrC1=C(C(=C(C(=C1)F)CC(=O)O)F)F
2-(4-bromo-2,3,6-trifluorophenyl)acetic acid; CAS No.: 537033-59-3; 2-(4-bromo-2,3,6-trifluorophenyl)acetic acid. PROPERTIES: This bromo-trifluoro-substituted phenylacetic acid features molecular formula C?H?BrF?O? with molecular weight 270.04 g/mol. It generally appears as a white crystalline powder. Soluble in non-polar and slightly polar organic solvents like hexanes and ethyl acetate. Melting point approximately 110-115 C. Exhibits IR absorption for carboxylic acid (~2900-2500 cm??) and C-Br/C-F groups (~600-500 cm?? and ~1300-1100 cm??). Thermogravimetric analysis indicates decomposition above 180 C under nitrogen. For optimal stability, store at 2-8 C in tightly sealed containers with desiccant, protected from light. The compound may cause severe skin burns and eye damage; therefore, rigorous containment and personal protection measures are essential during manipulation. APPLICATIONS: As a bromo-trifluoro-substituted phenylacetic acid, 2-(4-bromo-2,3,6-trifluorophenyl)acetic acid is predominantly utilized in the synthesis of kinase inhibitors. It serves as a key intermediate in constructing the core scaffold of these compounds, where the bromo and fluoro groups provide valuable binding affinity for the kinase active site as demonstrated in medicinal chemistry research (Journal of Medicinal Chemistry). Additionally, the compound participates in the development of fluorescent probes for bioimaging applications, where its carboxylic acid functionality enables conjugation to biomolecules via amide bond formation (Bioconjugate Chemistry). In materials science, it functions as a monomer for preparing polyurethane foams with enhanced thermal stability, where the bromo and fluoro substituents contribute to improved flame retardancy and mechanical properties (Polymer International).
Reviews
Write Your Own Review