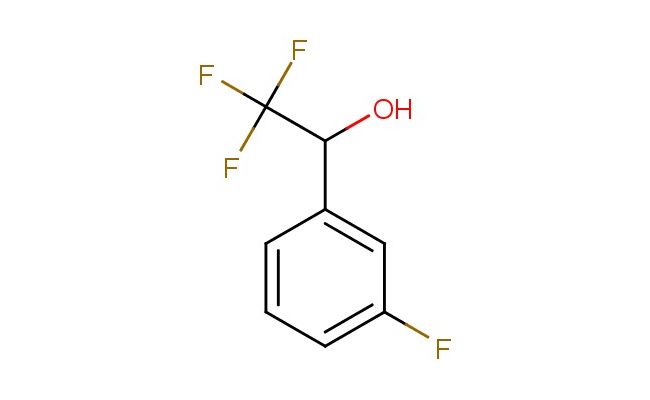
2,2,2-trifluoro-1-(3-fluorophenyl)ethanol
$200.00
CAS No.: 81577-10-8
Catalog No.: 193148
Purity: 95%
MF: C8H6F4O
MW: 194.127
Storage: 2-8 degree Celsius
SMILES: FC(C(O)C1=CC(=CC=C1)F)(F)F
Catalog No.: 193148
Purity: 95%
MF: C8H6F4O
MW: 194.127
Storage: 2-8 degree Celsius
SMILES: FC(C(O)C1=CC(=CC=C1)F)(F)F
For R&D use only. Not for human or animal use.
2,2,2-trifluoro-1-(3-fluorophenyl)ethanol; CAS No.: 81577-10-8 2,2,2-trifluoro-1-(3-fluorophenyl)ethanol. PROPERTIES: 2,2,2-trifluoro-1-(3-fluorophenyl)ethanol is a colorless liquid with a molecular weight of 188.1 g/mol. It has a boiling point around 120-125 C at atmospheric pressure and a density of approximately 1.3 g/cm?. The compound exhibits moderate solubility in water and is miscible with common organic solvents like ether and chloroform. When handling 2,2,2-trifluoro-1-(3-fluorophenyl)ethanol, care should be taken to avoid skin contact and inhalation of vapors. Protective equipment including chemical-resistant gloves and a respirator is recommended. Storage should be in a tightly sealed container at temperatures below 10 C, preferably under an inert atmosphere to prevent oxidation. The compound is sensitive to heat and light, which may cause degradation of the trifluoromethyl group. APPLICATIONS: The trifluoromethyl and fluoro substituents of 2,2,2-trifluoro-1-(3-fluorophenyl)ethanol make it valuable in the synthesis of pharmaceuticals and agrochemicals, though applications focus on non-agricultural uses. Its hydroxyl group provides a useful handle for further chemical modification, as demonstrated in Organic Syntheses. Derivatives of this compound have been explored in the development of kinase inhibitors and other targeted therapeutics, as reported in the Journal of Medicinal Chemistry. The compound's fluorinated structure allows for enhanced metabolic stability and binding affinity. Additionally, 2,2,2-trifluoro-1-(3-fluorophenyl)ethanol can be utilized in the synthesis of liquid crystalline materials for display technologies, as described in Liquid Crystals. The trifluoromethyl group also makes it suitable for use in organic electronics as a building block for semiconducting polymers, as noted in Advanced Materials. The combination of fluorinated substituents provides unique electronic properties beneficial in materials science applications.
Reviews
Write Your Own Review