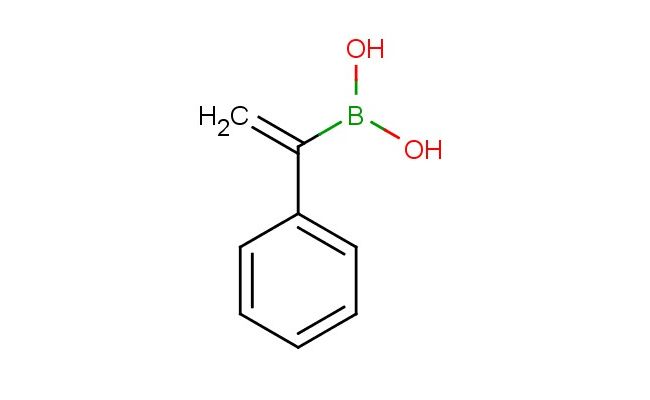
1-phenylvinylboronic acid
$385.00
CAS No.: 14900-39-1
Catalog No.: 196618
Purity: 95%
MF: C8H9BO2
MW: 147.97
Storage: 2-8 degree Celsius
SMILES: C1(=CC=CC=C1)C(=C)B(O)O
Catalog No.: 196618
Purity: 95%
MF: C8H9BO2
MW: 147.97
Storage: 2-8 degree Celsius
SMILES: C1(=CC=CC=C1)C(=C)B(O)O
1-phenylvinylboronic acid; CAS No.: 14900-39-1; 1-phenylvinylboronic acid. PROPERTIES: This boronic acid derivative exhibits molecular formula C8H7BO2 with molecular weight approximately 148.04 g/mol. It typically presents as white to off-white crystalline solid, demonstrating characteristic reactivity of boronic acid functional group. The compound shows moderate solubility in polar solvents like methanol and DMSO, while being sparingly soluble in non-polar hydrocarbons. Its melting point ranges between 142-146 C, and it exhibits IR absorption bands corresponding to the boronic acid group (~1200-1000 cm??) and aromatic C-H stretches. Thermogravimetric analysis reveals onset decomposition temperature above 200 C under nitrogen atmosphere. For optimal stability, 1-phenylvinylboronic acid should be stored at 2-8 C in tightly sealed amber glass containers, protected from moisture and prolonged light exposure. As with boronic acids, it may form covalent bonds with Lewis bases; therefore, standard laboratory safety precautions including nitrile gloves, safety goggles, and proper ventilation are recommended during handling. APPLICATIONS: The unique electronic properties of 1-phenylvinylboronic acid make it valuable as a building block in Suzuki-Miyaura cross-coupling reactions. It serves as a key intermediate in the preparation of biaryl compounds, particularly in constructing pharmaceutical scaffolds where the boronic acid group facilitates carbon-carbon bond formation between aromatic systems (Journal of Organic Chemistry). Additionally, the compound functions as a warhead in covalent inhibitor design, where it reacts with cysteine residues in target proteins as demonstrated in medicinal chemistry research (Bioorganic & Medicinal Chemistry Letters). The compound also finds utility in materials science as monomer for preparing polyboronic acid hydrogels with tunable mechanical properties, where its vinyl group participates in free radical polymerization reactions (Macromolecular Materials and Engineering).
Reviews
Write Your Own Review