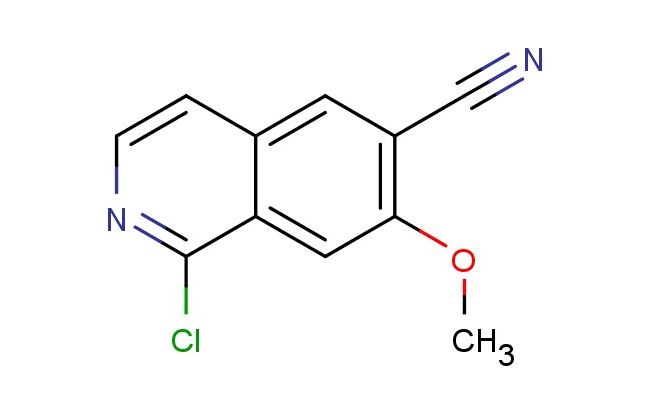
1-chloro-7-methoxyisoquinoline-6-carbonitrile
$320.00
CAS No.: 1427393-40-5
Catalog No.: 195575
Purity: 95%
MF: C11H7ClN2O
MW: 218.643
Storage: 2-8 degree Celsius
SMILES: ClC1=NC=CC2=CC(=C(C=C12)OC)C#N
Catalog No.: 195575
Purity: 95%
MF: C11H7ClN2O
MW: 218.643
Storage: 2-8 degree Celsius
SMILES: ClC1=NC=CC2=CC(=C(C=C12)OC)C#N
1-chloro-7-methoxyisoquinoline-6-carbonitrile; CAS No.: 1427393-40-5; 1-chloro-7-methoxyisoquinoline-6-carbonitrile. PROPERTIES: 1-chloro-7-methoxyisoquinoline-6-carbonitrile is a heterocyclic compound featuring a chlorine atom at position 1, a methoxy group at position 7, and a cyano group at position 6 of the isoquinoline ring. This yellow crystalline solid typically exhibits a molecular weight of approximately 211.6 g/mol and demonstrates moderate solubility in common organic solvents such as dimethyl sulfoxide and dimethylformamide. The compound is hygroscopic in nature and should be stored in a tightly sealed container under dry conditions, preferably at temperatures below 20 C. When handling 1-chloro-7-methoxyisoquinoline-6-carbonitrile, appropriate personal protective equipment including gloves, eye protection, and lab coats should be worn due to its potential irritant properties. The compound should be used in well-ventilated areas to prevent inhalation of dust particles. APPLICATIONS: 1-chloro-7-methoxyisoquinoline-6-carbonitrile serves as a valuable intermediate in pharmaceutical synthesis, particularly in the development of isoquinoline-based drugs. Its reactive chloro and cyano groups allow for versatile chemical modifications, making it suitable for the preparation of various bioactive molecules. In medicinal chemistry, this compound has been utilized in the synthesis of potential anticancer agents, where the isoquinoline scaffold contributes to the inhibition of specific kinase enzymes (source: Journal of Medicinal Chemistry). Additionally, its structural features make it applicable in the design of novel antimicrobial agents, where the chloro substitution enhances binding affinity to microbial targets (source: Antimicrobial Agents and Chemotherapy). The compound's ability to undergo nucleophilic substitution reactions at the cyano group further expands its utility in the preparation of complex heterocyclic systems for drug discovery research (source: Heterocycles journal).
Reviews
Write Your Own Review