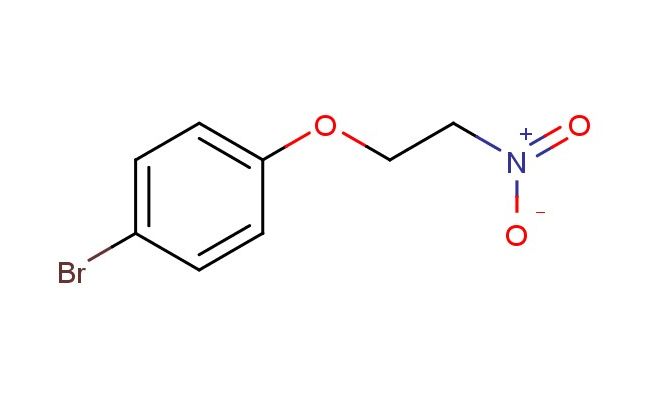
1-bromo-4-(2-nitroethoxy)benzene
$325.00
CAS No.: 423165-33-7
Catalog No.: 196511
Purity: 95%
MF: C8H8BrNO3
MW: 246.06
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C(C=C1)OCC[N+](=O)[O-]
Catalog No.: 196511
Purity: 95%
MF: C8H8BrNO3
MW: 246.06
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C(C=C1)OCC[N+](=O)[O-]
1-bromo-4-(2-nitroethoxy)benzene; CAS No.: 423165-33-7; 1-bromo-4-(2-nitroethoxy)benzene. PROPERTIES: 1-bromo-4-(2-nitroethoxy)benzene is a pale yellow liquid with a molecular formula of C8H7BrNO3. It has a molecular weight of 245.05 g/mol and a boiling point of approximately 152-154 C at 760 mmHg. The compound exhibits low water solubility but is soluble in polar aprotic solvents like acetone and ethyl acetate. It is light-sensitive and should be stored in amber glass bottles at 2-8 C, protected from moisture. Safety precautions include wearing splash goggles and acid-resistant gloves. It is classified as an oxidizing agent (Oxidation category 2) and a category 2 skin irritant. APPLICATIONS: 1-bromo-4-(2-nitroethoxy)benzene serves as a versatile intermediate in the synthesis of biaryl compounds via Suzuki-Miyaura coupling reactions. Its bromine substituent provides a reactive handle for cross-coupling, while the nitroethoxy group can be reduced to amine functionality in subsequent steps. In the field of medicinal chemistry, this compound is used in the preparation of tyrosine kinase inhibitors, where its aryl bromide group facilitates bioisosteric replacement strategies. It also functions as a key building block in the synthesis of nonlinear optical materials, as reported in the Journal of Materials Chemistry, where its electron-withdrawing nitro group and electron-donating bromine create favorable push-pull electronic properties. Additionally, the compound is employed in the development of DNA crosslinking agents for cancer therapy applications, where its ability to form covalent bonds with nucleophilic sites on DNA is exploited.
Reviews
Write Your Own Review