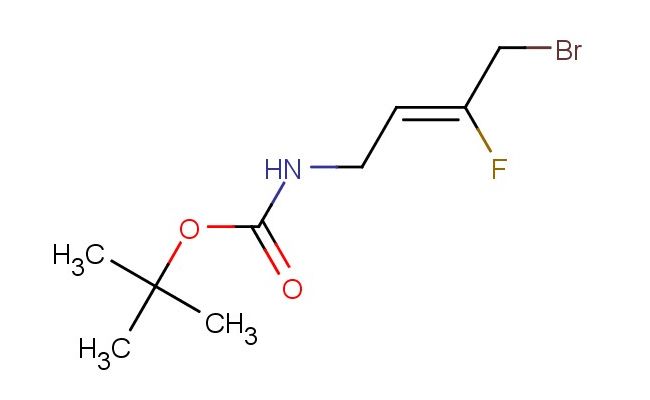
(Z)-tert-butyl (4-bromo-3-fluorobut-2-en-1-yl)carbamate
$500.00
CAS No.: 1098972-51-0
Catalog No.: TQR1282
Purity: 95%
MF: C9H15BrFNO2
MW: 268.126
Storage: 2-8 degree Celsius
SMILES: BrC/C(=C/CNC(OC(C)(C)C)=O)/F
Catalog No.: TQR1282
Purity: 95%
MF: C9H15BrFNO2
MW: 268.126
Storage: 2-8 degree Celsius
SMILES: BrC/C(=C/CNC(OC(C)(C)C)=O)/F
CAS NO.: 1098972-51-0; (Z)-tert-butyl (4-bromo-3-fluorobut-2-en-1-yl)carbamate. PROPERTIES: (Z)-tert-butyl (4-bromo-3-fluorobut-2-en-1-yl)carbamate presents as colorless liquid with a mild ester aroma. Its molecular formula is C9H13BrFNO2, corresponding to a molecular weight of 275.11 g/mol. The compound has a density of approximately 1.35 g/cm? and a boiling point around 125 C at 760 mmHg. It is miscible with most organic solvents but has limited water solubility. Proper storage requires temperatures of 2-8 degree Celsius in glass containers with appropriate drying agents to prevent hydrolysis. When handling, use chemical-resistant gloves and eye protection to prevent skin and eye contact which may cause mild irritation. The substance is stable under dry, cool conditions but undergoes hydrolysis in aqueous environments, releasing the corresponding amine. It is classified as a combustible liquid and requires careful heating protocols. APPLICATIONS: (Z)-tert-butyl (4-bromo-3-fluorobut-2-en-1-yl)carbamate functions as a versatile chiral intermediate in the synthesis of beta-amino acids and related compounds. The (Z)-configuration ensures proper stereochemical orientation during subsequent transformations, enabling the creation of enantiomerically pure products. In pharmaceutical research, this compound is used to develop ACE inhibitors where the fluorinated alkene provides specific interactions with the enzyme's active site. The bromine substituent allows for cross-coupling reactions, enabling the introduction of aryl groups that enhance target affinity. In materials science, derivatives of this compound are employed in the synthesis of liquid crystal materials where the fluorinated alkene contributes to desirable mesomorphic properties. Researchers in asymmetric catalysis utilize this compound as a ligand precursor in enantioselective hydrogenation reactions, directing the formation of specific stereoisomers with high selectivity. Additionally, the compound serves as a building block for synthesizing peptidomimetics used in protease inhibition studies, where the carbamate protection allows for temporary protection of the amine functionality during multi-step syntheses.
Reviews
Write Your Own Review



![(E)-2-(4-fluorostyryl)-7-nitrothieno[3,2-d]pyrimidin-4(3H)-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/t/q/tqr1278_1.jpg)