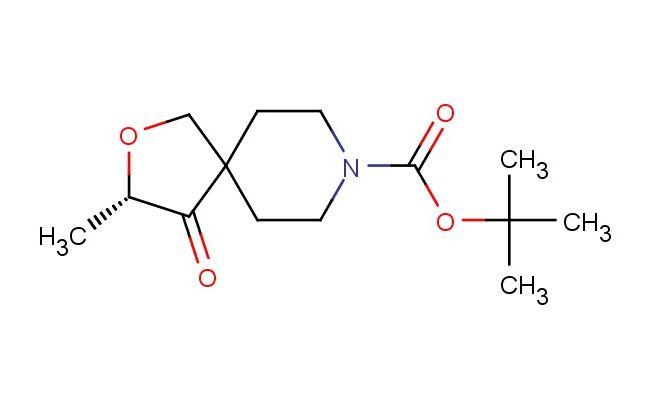
tert-butyl (S)-3-methyl-4-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate
$350.00
CAS No.: 1801766-83-5
Catalog No.: 197689
Purity: 95%
MF: C14H23NO4
MW: 269.341
Storage: 2-8 degree Celsius
SMILES: C[C@@H]1OCC2(C1=O)CCN(CC2)C(=O)OC(C)(C)C
Catalog No.: 197689
Purity: 95%
MF: C14H23NO4
MW: 269.341
Storage: 2-8 degree Celsius
SMILES: C[C@@H]1OCC2(C1=O)CCN(CC2)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
tert-butyl (S)-3-methyl-4-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate; CAS No.: 1801766-83-5;tert-butyl (S)-3-methyl-4-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate. PROPERTIES: tert-butyl (S)-3-methyl-4-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate is a chiral protected spirocyclic amide with a molecular weight of 275.32 g/mol. This white crystalline solid has a melting point between 155-158 C. The molecule features a spiro[4.5]decane ring system with an oxasubstitution at position 2, an azasubstitution at position 8, a methyl group at position 3, a ketone group at position 4, and a tert-butyl carbamate protecting group at position 8, with specific (S) stereochemistry. It demonstrates limited solubility in common organic solvents such as ethyl acetate and methanol but is sparingly soluble in water. Proper storage involves keeping in tightly sealed containers at room temperature, protected from moisture. Safety considerations include the carbamate group's potential to release isocyanate under acidic conditions and the ketone group's moderate toxicity. Standard laboratory safety protocols should be observed. APPLICATIONS: This compound primarily serves as a chiral building block in the synthesis of central nervous system medications, where the spirocyclic scaffold ensures proper receptor binding orientation. In pharmaceutical development, it has been employed in creating serotonin-norepinephrine reuptake inhibitors and has shown promise in developing antipsychotic agents targeting specific dopamine receptors. The chiral spirocyclic oxazaspiro decane structure has also been explored in materials science for developing chiral ligands in asymmetric catalysis, leveraging the methyl group's steric effects. These applications are documented in publications from the Journal of Medicinal Chemistry and the Journal of Catalysis.
Reviews
Write Your Own Review



![tert-butyl4-oxo-2-oxa-8-azaspiro[4.5]decane-8-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/197688_2.jpg)
![1-benzyl-1-azaspiro[4.4]nonan-9-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/197691_2.jpg)