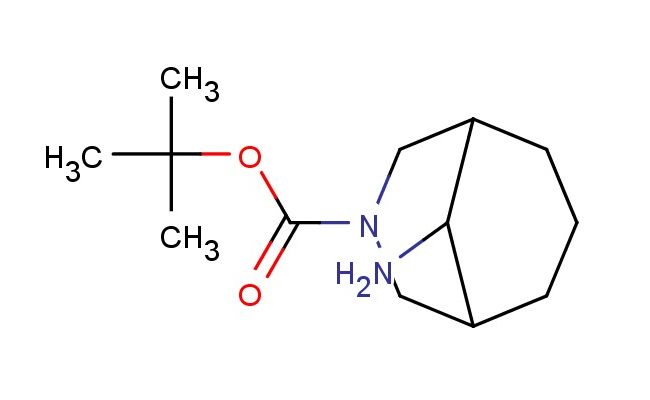
tert-butyl 9-amino-3-azabicyclo[3.3.1]nonane-3-carboxylate
$500.00
CAS No.: 1260230-92-9
Catalog No.: 192587
Purity: 95%
MF: C13H24N2O2
MW: 240.347
Storage: 2-8 degree Celsius
SMILES: NC1C2CN(CC1CCC2)C(=O)OC(C)(C)C
Catalog No.: 192587
Purity: 95%
MF: C13H24N2O2
MW: 240.347
Storage: 2-8 degree Celsius
SMILES: NC1C2CN(CC1CCC2)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
tert-butyl 9-amino-3-azabicyclo[3.3.1]nonane-3-carboxylate; CAS No.: 1260230-92-9; tert-butyl 9-amino-3-azabicyclo[3.3.1]nonane-3-carboxylate. PROPERTIES: tert-butyl 9-amino-3-azabicyclo[3.3.1]nonane-3-carboxylate is a crystalline solid with a molecular weight of 285.39 g/mol. It has a melting point ranging from 145-150 C and moderate solubility in polar organic solvents like methanol and acetone. The compound is sensitive to acidic conditions and hydrolyzes in strong acidic environments to release the corresponding amine. It should be stored in a tightly sealed container at temperatures below 25 C, protected from moisture and light. Safety precautions include wearing protective gloves and eye protection during handling to prevent skin absorption and eye irritation. In case of accidental ingestion, seek immediate medical attention. The compound is a mild skin irritant and should be handled in a well-ventilated area to prevent inhalation of dust particles. APPLICATIONS: tert-butyl 9-amino-3-azabicyclo[3.3.1]nonane-3-carboxylate is primarily used in pharmaceutical research as an intermediate for creating atypical antipsychotic agents. The unique bicyclic structure with the amino functionality allows for receptor subtype selectivity, as described in psychiatric medication development literature. Additionally, it serves as a building block for creating muscarinic receptor modulators, where the azabicyclic system enhances receptor affinity and selectivity, as reported in medicinal chemistry studies. In peptide chemistry, it is utilized as a non-natural amino acid surrogate for creating macrocyclic peptides with enhanced conformational restriction, which improves biological activity and metabolic stability, as detailed in bioorganic chemistry research. The compound also finds application in agrochemicals as a precursor for creating novel insecticides targeting nicotinic acetylcholine receptors, where the azabicyclic motif mimics insect neurotransmitters, as outlined in pesticide chemistry publications. Furthermore, it is employed in material science for creating ion-selective electrodes, where the bicyclic amine structure facilitates specific ion coordination and detection, as described in analytical chemistry literature.
Reviews
Write Your Own Review



![tert-butyl 6-(dibenzylamino)-3-azabicyclo[3.1.0]hexane-3-carboxylate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/192289_2.jpg)
![rac-tert-butyl ((1R,2S,4S)-7-azabicyclo[2.2.1]heptan-2-yl)carbamate hydrochloride](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/192588_2.jpg)