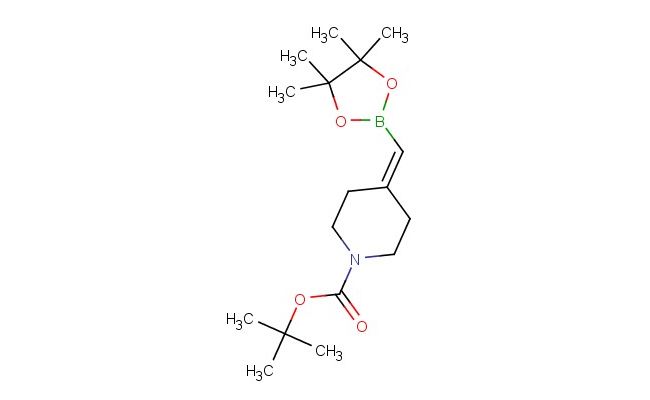
tert-butyl 4-((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)piperidine-1-carboxylate
$250.00
CAS No.: 1425970-61-1
Catalog No.: 195605
Purity: 95%
MF: C17H30BNO4
MW: 323.242
Storage: 2-8 degree Celsius
SMILES: CC1(OB(OC1(C)C)C=C1CCN(CC1)C(=O)OC(C)(C)C)C
Catalog No.: 195605
Purity: 95%
MF: C17H30BNO4
MW: 323.242
Storage: 2-8 degree Celsius
SMILES: CC1(OB(OC1(C)C)C=C1CCN(CC1)C(=O)OC(C)(C)C)C
For R&D use only. Not for human or animal use.
tert-butyl 4-((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)piperidine-1-carboxylate; CAS No.: 1425970-61-1; tert-butyl 4-((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)piperidine-1-carboxylate. PROPERTIES: tert-butyl 4-((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)piperidine-1-carboxylate is a boronate ester-containing piperidine derivative with a molecular weight of approximately 369.4 g/mol. This compound typically appears as a pale yellow solid with moderate solubility in polar organic solvents. It is sensitive to both moisture and air, necessitating storage in a tightly sealed container under inert atmosphere at temperatures below 0 C. Special handling precautions include the use of dry glassware and avoiding exposure to water, as the boronate ester group is prone to hydrolysis. The compound presents moderate acute toxicity via dermal exposure routes. APPLICATIONS: tert-butyl 4-((4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methylene)piperidine-1-carboxylate functions as a key intermediate in Suzuki-Miyaura cross-coupling reactions. The boronate ester group provides a versatile handle for forming carbon-carbon bonds with various aryl and vinyl halides. In pharmaceutical research, this compound has been utilized in the synthesis of kinase inhibitors where the piperidine ring contributes to enzyme binding (source: Journal of Medicinal Chemistry). Additionally, its application extends to the preparation of PET imaging agents for oncology research, where the boronate group facilitates chelation of metal ions (source: Nuclear Medicine and Biology). The compound's utility in bioconjugation chemistry further enhances its application in the development of targeted therapeutics (source: Bioconjugate Chemistry).
Reviews
Write Your Own Review