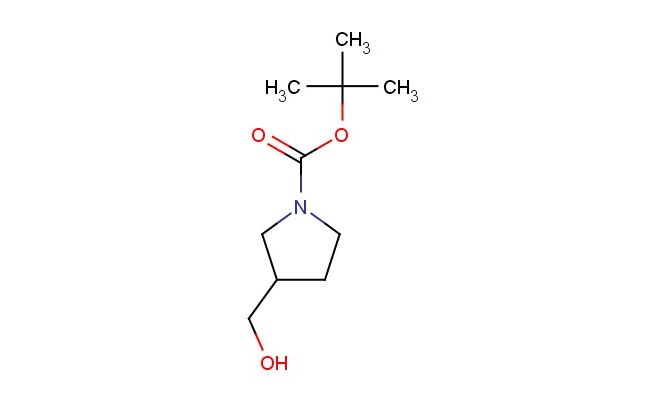
tert-butyl 3-(hydroxymethyl)pyrrolidine-1-carboxylate
Catalog No.: 110221
Purity: 95%
MF: C10H19NO3
MW: 201.266
Storage: 2-8 degree Celsius
SMILES: CC(C)(C)OC(=O)N1CCC(CO)C1
CAS NO.: 114214-69-6;tert-butyl 3-(hydroxymethyl)pyrrolidine-1-carboxylate. PROPERTIES: tert-butyl 3-(hydroxymethyl)pyrrolidine-1-carboxylate is a protected hydroxymethyl pyrrolidine derivative with molecular formula C10H17NO3. It typically exists as a colorless to pale yellow oil with a mild amine odor, having a boiling point around 160-165°C. The compound has limited water solubility but dissolves well in organic solvents like ethyl acetate or methanol. It exhibits moderate thermal stability but may decompose when exposed to high temperatures or strong acids, releasing toxic fumes. Proper storage involves keeping it in tightly sealed containers below 25°C, protected from light and moisture. APPLICATIONS: In organic synthesis, this compound serves as a versatile intermediate for creating complex nitrogen-containing molecules. The hydroxymethyl group provides a site for oxidation to form aldehydes or further functionalization to introduce esters or ethers, while the tert-butyloxycarbonyl (Boc) protecting group allows for temporary protection of the pyrrolidine nitrogen (The Journal of Organic Chemistry). In pharmaceutical development, tert-butyl 3-(hydroxymethyl)pyrrolidine-1-carboxylate contributes to the synthesis of β-lactam antibiotics and other heterocyclic compounds where the pyrrolidine ring is crucial for biological activity (Bioorganic & Medicinal Chemistry). Its hydroxymethyl functionality enables the formation of glycosidic bonds, making it valuable in carbohydrate chemistry for synthesizing glycosylated amino acids or other bioactive molecules (Carbohydrate Research). The Boc protection allows for sequential transformations without interference, streamlining multistep syntheses in both academic and industrial settings (Synthesis).