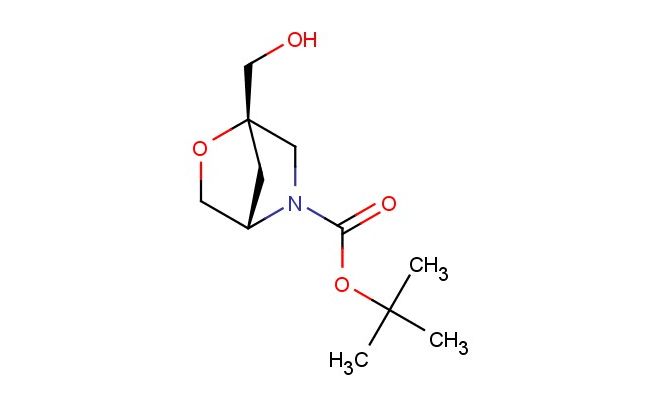
tert-butyl (1S,4S)-1-(hydroxymethyl)-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate
$1,800.00
CAS No.: 2306248-41-7
Catalog No.: 200356
Purity: 95%
MF: C11H19NO4
MW: 229.276
Storage: 2-8 degree Celsius
SMILES: OC[C@@]12OC[C@@H](N(C1)C(=O)OC(C)(C)C)C2
Catalog No.: 200356
Purity: 95%
MF: C11H19NO4
MW: 229.276
Storage: 2-8 degree Celsius
SMILES: OC[C@@]12OC[C@@H](N(C1)C(=O)OC(C)(C)C)C2
For R&D use only. Not for human or animal use.
CAS NO.: 2306248-41-7;tert-butyl (1S,4S)-1-(hydroxymethyl)-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate. PROPERTIES: This chiral compound presents as a white to off-white crystalline powder with limited aqueous solubility but good dissolution in polar organic solvents such as methanol and acetonitrile. The tert-butyl (1S,4S)-1-(hydroxymethyl)-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate exhibits a molecular weight of approximately 247.3 g/mol, featuring a strained bicyclic framework with multiple stereocenters. Stability assessments reveal sensitivity to acidic conditions and elevated temperatures, mandating storage between 2-8 degree Celsius in amber glass containers with desiccants. When handling, personnel should utilize powder hoods, nitrile gloves, and protective eyewear. In case of inhalation, move to fresh air and administer oxygen if needed. Skin contact requires immediate washing with soap and water, while eye exposure necessitates 15 minutes of rinsing followed by medical evaluation. Spills should be addressed with absorbent materials and disposed of as hazardous waste. APPLICATIONS: The tert-butyl (1S,4S)-1-(hydroxymethyl)-2-oxa-5-azabicyclo[2.2.1]heptane-5-carboxylate serves as a critical chiral building block in asymmetric synthesis pathways, particularly valuable in the construction of macrocyclic peptides and complex heterocycles. Its unique bicyclic architecture provides conformational restriction that enhances binding affinity in medicinal chemistry applications. Research institutions employ this compound as a precursor to bioisosteres in lead optimization campaigns. Additionally, its oxabicyclic motif finds utility in the development of enzyme inhibitors where precise spatial arrangement of functional groups is crucial. The compound's strategic positioning in synthetic routes allows for diverse transformations including hydrogenolysis and nucleophilic substitution, extending its application in the preparation of specialized pharmaceutical intermediates and diagnostic agents.
Reviews
Write Your Own Review



![endo-3-Boc-3-azabicyclo[3.1.0]hexane-6-acetic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/7/171311.jpg)
![3-(4-hydroxyphenyl)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2,4-dione](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/x/h/xh0786.jpg)