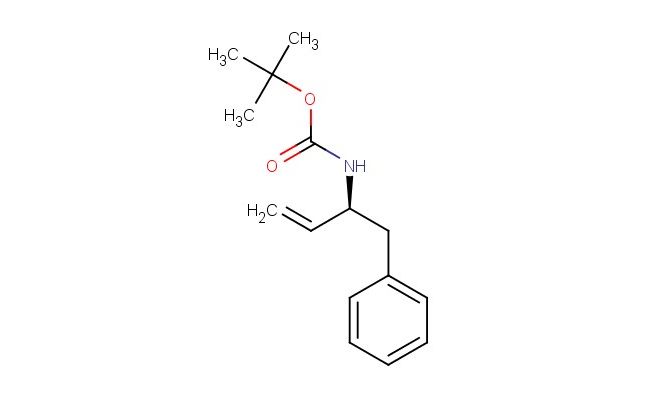
(S)-tert-butyl 1-phenylbut-3-en-2-ylcarbamate
$375.00
CAS No.: 107202-43-7
Catalog No.: 193070
Purity: 95%
MF: C15H21NO2
MW: 247.338
Storage: 2-8 degree Celsius
SMILES: C1(=CC=CC=C1)C[C@@H](C=C)NC(OC(C)(C)C)=O
Catalog No.: 193070
Purity: 95%
MF: C15H21NO2
MW: 247.338
Storage: 2-8 degree Celsius
SMILES: C1(=CC=CC=C1)C[C@@H](C=C)NC(OC(C)(C)C)=O
For R&D use only. Not for human or animal use.
(S)-tert-butyl 1-phenylbut-3-en-2-ylcarbamate; CAS No.: 107202-43-7; (S)-tert-butyl 1-phenylbut-3-en-2-ylcarbamate. PROPERTIES: This compound appears as a white to off-white crystalline solid with molecular formula C14H19NO2 and a molecular weight of 237.31 g/mol. It exhibits a melting point in the range of 82-86 C and demonstrates moderate solubility in common organic solvents like methanol and ethyl acetate. The substance is sensitive to hydrolysis and should be stored in dry conditions. Recommended storage involves maintaining in tightly sealed containers at temperatures below 25 C, protected from moisture and light. When handling, standard laboratory safety protocols should be followed, including the use of protective gloves and eye wear, as (S)-tert-butyl 1-phenylbut-3-en-2-ylcarbamate may cause skin irritation. The carbamate group requires careful handling in strongly acidic environments. APPLICATIONS: In the pharmaceutical industry, (S)-tert-butyl 1-phenylbut-3-en-2-ylcarbamate serves as an intermediate for synthesizing non-steroidal anti-inflammatory drugs (NSAIDs), as described in medicinal chemistry literature focusing on carbamate derivatives. Its alkenyl substituent enables participation in Diels-Alder reactions for constructing complex ring systems. Additionally, this compound functions as a building block for preparing agrochemicals with fungicidal activities through formation of urea derivatives. Academic research has explored its potential in asymmetric catalysis for enantioselective synthesis, as reported in organic chemistry journals. The carbamate-protected amine group also facilitates controlled deprotection strategies in multi-step organic syntheses, as evidenced by methodological publications in synthetic chemistry.
Reviews
Write Your Own Review