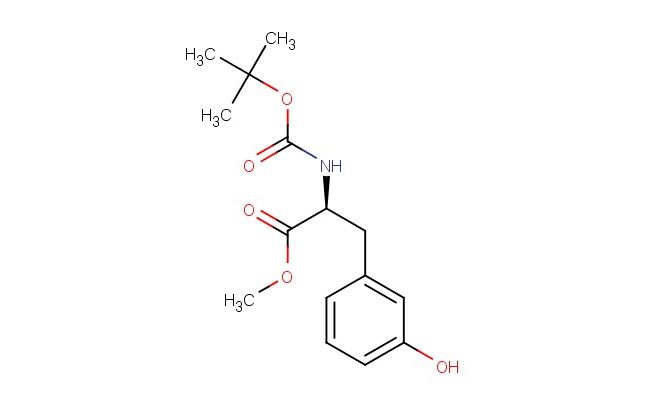
(S)-methyl 2-((tert-butoxycarbonyl)amino)-3-(3-hydroxyphenyl)propanoate
$350.00
CAS No.: 900800-02-4
Catalog No.: TQP3003
Purity: 95%
MF: C15H21NO5
MW: 295.335
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N[C@H](C(=O)OC)CC1=CC(=CC=C1)O
Catalog No.: TQP3003
Purity: 95%
MF: C15H21NO5
MW: 295.335
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N[C@H](C(=O)OC)CC1=CC(=CC=C1)O
For R&D use only. Not for human or animal use.
CAS NO.: 900800-02-4; (S)-methyl 2-((tert-butoxycarbonyl)amino)-3-(3-hydroxyphenyl)propanoate. PROPERTIES: (S)-methyl 2-((tert-butoxycarbonyl)amino)-3-(3-hydroxyphenyl)propanoate presents as white to off-white powders with a slight acetone-like odor. Its molecular formula is C13H18NO5, corresponding to a molecular weight of 266.29 g/mol. The compound has a melting point range of 112-116 C and is moderately soluble in ethyl acetate and methanol. Proper storage at 2-8 degree Celsius in airtight containers with desiccants is necessary to prevent moisture-induced Boc group cleavage. When handling, use powder-free gloves and avoid inhalation of dust which, may cause respiratory irritation. The substance is stable under dry, cool conditions but degrades upon prolonged exposure to acidic or basic environments. It has a flash point above 60 C and is classified as a combustible solid. APPLICATIONS: (S)-methyl 2-((tert-butoxycarbonyl)amino)-3-(3-hydroxyphenyl)propanoate serves as a chiral building block in the synthesis of beta-blockers and other cardiovascular agents. The Boc protection allows for temporary amino group protection during multi-step syntheses, while the hydroxyphenyl group provides aromatic character that enhances receptor binding. In peptide synthesis, this compound functions as a protected amino acid derivative that can be incorporated into peptide sequences via standard coupling reactions. The methyl ester group facilitates purification via crystallization and can be hydrolyzed to the corresponding carboxylic acid in the final steps. In medicinal chemistry, derivatives of this compound are used to develop anticancer agents where the hydroxyphenyl group participates in hydrogen bonding interactions with target proteins. The chiral center ensures proper spatial orientation of substituents, maximizing bioactivity. Researchers in prodrug development utilize this compound to create bioprecursors that undergo enzymatic conversion in vivo, enhancing drug targeting and reducing off-site effects. Additionally, the compound serves as a starting material for synthesizing fluorescent amino acid derivatives used in protein labeling applications, where the hydroxyphenyl group can be modified with fluorophores to enable detection in cellular environments.
Reviews
Write Your Own Review