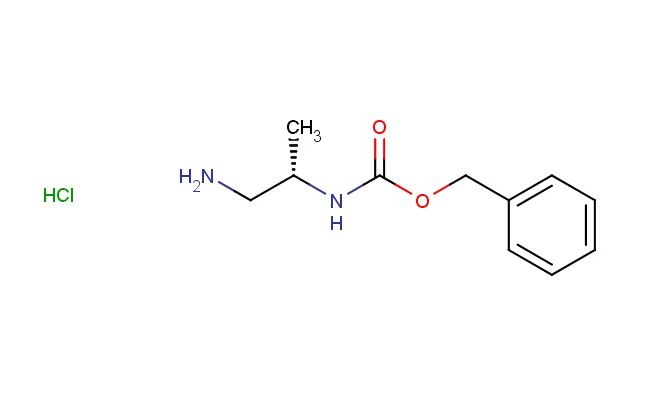
(S)-benzyl 1-aminopropan-2-ylcarbamate hydrochloride
$225.00
CAS No.: 850033-71-5
Catalog No.: 193150
Purity: 95%
MF: C11H17ClN2O2
MW: 244.722
Storage: 2-8 degree Celsius
SMILES: Cl.NC[C@H](C)NC(OCC1=CC=CC=C1)=O
Catalog No.: 193150
Purity: 95%
MF: C11H17ClN2O2
MW: 244.722
Storage: 2-8 degree Celsius
SMILES: Cl.NC[C@H](C)NC(OCC1=CC=CC=C1)=O
For R&D use only. Not for human or animal use.
(S)-benzyl 1-aminopropan-2-ylcarbamate hydrochloride; CAS No.: 850033-71-5 (S)-benzyl 1-aminopropan-2-ylcarbamate hydrochloride. PROPERTIES: (S)-benzyl 1-aminopropan-2-ylcarbamate hydrochloride is a crystalline powder with a molecular weight of 253.8 g/mol. It has a melting point between 170-175 C (decomposition) and is highly soluble in water. The compound exhibits typical amine reactivity and should be handled with care to prevent exposure to moisture, as the hydrochloride salt is hygroscopic. When working with (S)-benzyl 1-aminopropan-2-ylcarbamate hydrochloride, protective equipment including chemical-resistant gloves and a respirator is recommended. Storage should be in a tightly sealed container at 2-8 C, preferably under anhydrous conditions. The compound is sensitive to light and should be protected from direct sunlight. APPLICATIONS: The chiral structure of (S)-benzyl 1-aminopropan-2-ylcarbamate hydrochloride makes it valuable in asymmetric synthesis and peptide chemistry. Its carbamate functionality allows for controlled release of the amine group upon deprotection, as demonstrated in Organic Syntheses. Derivatives of this compound have been explored in the development of beta-blockers and other cardiovascular pharmaceuticals, as reported in the Journal of Medicinal Chemistry. The benzyl protection provides a convenient handle for further functionalization in synthetic sequences. Additionally, the compound can be utilized in the preparation of chiral catalysts and ligands for asymmetric catalysis, as highlighted in Advanced Synthesis & Catalysis. The hydrochloride salt form helps stabilize the compound but requires careful handling to avoid deliquescence. The combination of the benzyl and carbamate protections makes this compound particularly useful in solid-phase synthesis and the preparation of combinatorial libraries for drug discovery, as described in the Journal of Combinatorial Chemistry.
Reviews
Write Your Own Review