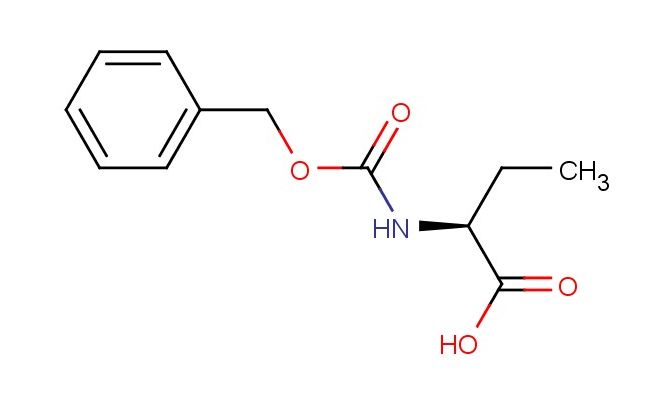
(S)-2-(((benzyloxy)carbonyl)amino)butanoic acid
$300.00
CAS No.: 42918-86-5
Catalog No.: 196558
Purity: 95%
MF: C12H15NO4
MW: 237.255
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC(=O)N[C@H](C(=O)O)CC
Catalog No.: 196558
Purity: 95%
MF: C12H15NO4
MW: 237.255
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC(=O)N[C@H](C(=O)O)CC
For R&D use only. Not for human or animal use.
(S)-2-(((benzyloxy)carbonyl)amino)butanoic acid; CAS No.: 42918-86-5; (S)-2-(((benzyloxy)carbonyl)amino)butanoic acid. PROPERTIES: This amino acid derivative is a white crystalline substance with molecular formula C11H15NO4. It has molecular weight of 229.24 g/mol and typically melts at 145-150 C. The compound is moderately soluble in polar solvents and exhibits hygroscopic tendencies, necessitating storage in desiccator at 2-8 C. As with many carbamate-protected amines, it may release carbon dioxide upon acidic hydrolysis; fume hoods should be used during such reactions. APPLICATIONS: In peptide synthesis, this compound serves as protected amino acid building block for constructing bioactive peptides. The (S)-configuration matches natural amino acid stereochemistry, allowing for native peptide bond formation (Journal of Peptide Research). The benzyloxycarbonyl (Z) group provides temporary protection of the alpha-amino group, which can be removed by hydrogenolysis using palladium catalyst. Additionally, it functions as intermediate for producing beta-lactam antibiotics where the four-membered lactam ring is constructed from this amino acid precursor (Journal of Antibiotics). The carboxylic acid group enables coupling reactions with various amines and hydroxyl-containing compounds using standard peptide coupling reagents like EDC or HATU.
Reviews
Write Your Own Review