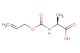
(S)-2-(((allyloxy)carbonyl)amino)-3-methylbutanoic acid
$150.00
CAS No.: 115491-96-8
Catalog No.: TQP2372
Purity: 95%
MF: C9H15NO4
MW: 201.222
Storage: 2-8 degree Celsius
SMILES: C(C=C)OC(=O)N[C@H](C(=O)O)C(C)C
Catalog No.: TQP2372
Purity: 95%
MF: C9H15NO4
MW: 201.222
Storage: 2-8 degree Celsius
SMILES: C(C=C)OC(=O)N[C@H](C(=O)O)C(C)C
For R&D use only. Not for human or animal use.
CAS NO.: 115491-96-8; (S)-2-(((allyloxy)carbonyl)amino)-3-methylbutanoic acid. PROPERTIES: (S)-2-(((allyloxy)carbonyl)amino)-3-methylbutanoic acid appears as white crystalline solids with a characteristic carboxylic acid odor. Its molecular formula is C9H15NO4, with a molecular weight of 193.22 g/mol. The compound has a melting point between 82-86 C and a pKa value of approximately 2.89. It is moderately soluble in water and highly soluble in polar organic solvents like methanol and acetonitrile. Proper storage requires temperatures of 2-8 degree Celsius in glass containers with Teflon-lined caps to prevent degradation. When handling, use corrosion-resistant equipment as it may cause severe skin burns and eye damage. The substance is stable under dry conditions but hydrolyzes in aqueous solutions, releasing the corresponding amine and allyl alcohol. It is sensitive to strong acids and bases, which catalyze its decomposition. APPLICATIONS: (S)-2-(((allyloxy)carbonyl)amino)-3-methylbutanoic acid serves as a chiral building block in peptide synthesis. The allyloxycarbonyl (Alloc) protection group allows for selective deprotection using palladium-catalyzed hydrogenolysis, enabling orthogonal protection strategies in complex peptide assemblies. In medicinal chemistry, this compound is used to synthesize angiotensin-converting enzyme (ACE) inhibitors where the branched alkyl group enhances binding affinity to the active site. The chiral center ensures proper orientation within the binding pocket, maximizing inhibitory activity. In biocatalysis, derivatives of this compound are employed as cofactors in enzyme-catalyzed resolutions, enhancing enantioselectivity in kinetic resolution processes. Additionally, the compound functions as a starting material for synthesizing beta-amino acids with specific configurations, valuable in the development of peptidomimetics with improved metabolic stability. Researchers in protein engineering utilize this compound to introduce non-natural amino acids into recombinant proteins, enabling the study of structure-function relationships through site-specific modifications. The Alloc protection provides a means of temporary protection during multi-step protein semisynthesis operations.
Reviews
Write Your Own Review