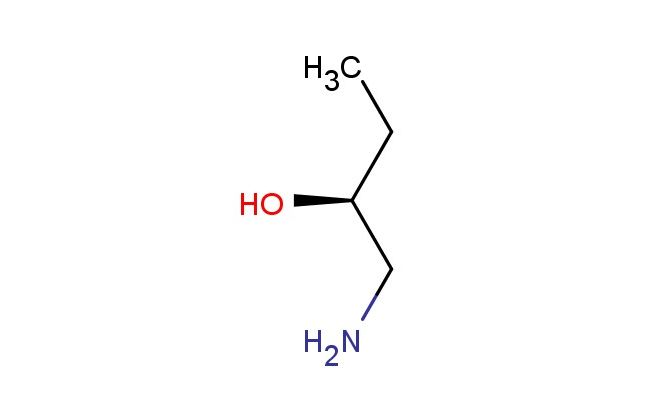
(S)-1-Amino-2-butanol
$500.00
CAS No.: 30608-63-0
Catalog No.: 200467
Purity: 95%
MF: C4H11NO
MW: 89.138
Storage: 2-8 degree Celsius
SMILES: NC[C@H](CC)O
Catalog No.: 200467
Purity: 95%
MF: C4H11NO
MW: 89.138
Storage: 2-8 degree Celsius
SMILES: NC[C@H](CC)O
CAS NO.: 30608-63-0;(S)-1-Amino-2-butanol. PROPERTIES: This chiral alcoholamine presents as a colorless liquid with a mild amine odor, exhibiting a molecular weight of approximately 103.13 g/mol. The (S)-1-Amino-2-butanol demonstrates good solubility in water and polar organic solvents such as ethanol and acetone. Stability assessments reveal sensitivity to oxidative degradation when exposed to air for extended periods and requires storage between 2-8 degree Celsius in tightly sealed containers. When handling this compound, protective equipment including nitrile gloves, safety goggles, and adequate ventilation should be utilized. In case of skin contact, wash thoroughly with soap and water; for eye exposure, rinse with water for at least 15 minutes and seek medical attention. Inhalation of vapors may cause respiratory tract irritation, and adequate ventilation should be maintained. Spills should be cleaned using absorbent materials and disposed of according to local regulations. APPLICATIONS: The (S)-1-Amino-2-butanol serves as a valuable chiral building block in the synthesis of various pharmaceuticals, particularly in the production of beta-blockers and antiviral agents where its chiral amine-alcohol framework provides essential steric and electronic properties. Research laboratories utilize this compound as a starting material for the preparation of asymmetric catalysts, which are crucial in the synthesis of enantiomerically pure compounds. Additionally, its chiral primary amine and secondary alcohol functionalities make it suitable for the synthesis of complex molecules such as glycosides and nucleoside analogs. The compound's versatility is further enhanced by its ability to undergo various chemical reactions, including esterification, amidation, and reductive amination, making it an important intermediate in both academic research and industrial chemical production.
Reviews
Write Your Own Review